Abstract
SUMMARY: Identification of carotid artery atherosclerosis is conventionally based on measurements of luminal stenosis and surface irregularities using in vivo imaging techniques including sonography, CT and MR angiography, and digital subtraction angiography. However, histopathologic studies demonstrate considerable differences between plaques with identical degrees of stenosis and indicate that certain plaque features are associated with increased risk for ischemic events. The ability to look beyond the lumen using highly developed vessel wall imaging methods to identify plaque vulnerable to disruption has prompted an active debate as to whether a paradigm shift is needed to move away from relying on measurements of luminal stenosis for gauging the risk of ischemic injury. Further evaluation in randomized clinical trials will help to better define the exact role of plaque imaging in clinical decision-making. However, current carotid vessel wall imaging techniques can be informative. The goal of this article is to present the perspective of the ASNR Vessel Wall Imaging Study Group as it relates to the current status of arterial wall imaging in carotid artery disease.
ABBREVIATIONS:
- CE
- contrast-enhanced
- DIR
- double inversion recovery
- FC
- fibrous cap
- IPH
- intraplaque hemorrhage
- IVUS
- intravascular ultrasound
- LRNC
- lipid-rich necrotic core
- MATCH
- multicontrast atherosclerosis characterization
- SNAP
- simultaneous noncontrast angiography and intraplaque hemorrhage
- SPACE
- sampling perfection with application-optimized contrasts using different flip angle evolution
- US
- ultrasound
The word “atherosclerosis” is derived from the 2 Greek words “athera” (mush) and “sclerosis” (hardening), indicating hardening of the vascular wall. This disease is highly prevalent in developed countries, with carotid artery narrowing reported in up to 75% of men and 62% of women 65 years of age and older.1 Stroke is the second most common cause of death worldwide,2 and approximately 18%–25% of all strokes are due to carotid atherosclerotic disease.3
Conventionally, identification of atherosclerosis affecting the carotid artery is based on measurements of the degree of luminal stenosis and surface irregularities4,5 by sonography, catheter-based angiography, and, nowadays, CTA or MRA.6,7 However, histopathologic studies initially performed on coronary arteries and subsequently on carotid arteries demonstrate considerable differences between plaques with identical degrees of stenosis. These observations led to research indicating that certain plaque features are associated with increased risk for ischemic events.8⇓–10 The more recent introduction of fast multidetector row CT technology, high-field MR imaging, and advanced ultrasound (US) systems has enabled accurate characterization of plaque features that relate to risk of ischemic injury.11⇓⇓–14 The ability to look beyond the lumen using advanced wall imaging methods to identify “vulnerable plaque”15,16 is spurring a paradigm shift away from simple measurement of percent luminal stenosis for gauging the risk of ischemic injury. Currently, characterization of the vessel wall and atherosclerotic plaque is the focus of several ongoing research studies that are investigating the optimal approach to vulnerable plaque imaging.17⇓–19
Further evaluation in randomized clinical trials is needed to establish the exact role of plaque imaging in clinical decision-making. However, carotid vessel wall imaging techniques may be beneficial at present. For example, improved visualization of the location and extent of atherosclerotic plaque would assist in surgical planning before endarterectomy or carotid artery stent placement. Vessel wall imaging may also be helpful in borderline clinical cases. Identification of carotid plaque harboring a large lipid-rich necrotic core (LRNC) with ulceration and intraplaque hemorrhage (IPH) despite guideline-based medical therapy in a patient with repeat strokes and 50% carotid stenosis may lead to consideration for carotid endarterectomy. In asymptomatic patients, vessel wall imaging with a large LRNC may represent the phenotype of atherosclerotic disease amenable to more intensive lipid-lowering therapy.20 Similarly, progressive vulnerable plaque features with increasing IPH in asymptomatic carotid stenosis may benefit from more intensive lipid-lowering therapy.21,22
The goal of this article is therefore to present the perspective of the ASNR Vessel Wall Imaging Study Group on the current status of arterial wall imaging in carotid artery disease.
Clinical Background and Physiology
For several years, digital subtraction angiography remained the primary imaging method for studying carotid arteries for detecting stenosis secondary to atherosclerotic plaque.23,24 The method provides optimal spatial resolution for defining the opacified lumen, the associated degree of luminal stenosis, and plaque-related luminal changes that include lumen irregularity and plaque ulcerations.25,26
Carotid endarterectomy trials were undertaken during the 1980s to mid-1990s that quantified the benefit of carotid endarterectomy according to the degree of luminal stenosis.4,27⇓⇓–30 These studies became the basis for considering degree of stenosis as the primary metric for stratifying subsequent stroke risk and selecting the optimal therapeutic approach (surgery versus best medical management).27 In particular, 3 multicenter randomized studies, the European Carotid Surgery Trial (ECST), NASCET, and Asymptomatic Carotid Atherosclerosis Study (ACAS) evaluated cutoffs for the degree of carotid stenosis as they relate to stroke risk reduction by carotid endarterectomy.28⇓–30
NASCET, ECST, and ACAS used DSA to assess the percent reduction in the luminal diameter of the artery. The methodology for carotid stenosis quantification is debated because NASCET and ECST used indirect ratio-percent methods.31 Stenosis measurements with NASCET and ECST differ substantially: With the ECST method, twice as many stenoses were classified as severe, and less than one-third of the number of stenoses, as mild compared with the NASCET method.32 Techniques that enable identification of both outer and inner walls of the artery might lead to more accurate assessment of risk. Bartlett et al33 suggested the use of this direct diameter–based measurement to overcome the limitations of the percent-based methods, and the results they found suggest that this technique could be efficient.
The degree of luminal stenosis as a marker of atherosclerotic disease severity has been criticized because of observations that plaques producing only mild-to-moderate stenosis may still lead to acute cerebral infarction.34⇓⇓⇓⇓⇓⇓–41 Histopathologic evaluation of these plaques showed that plaque erosion and disruption were common morphologic features found in symptomatic lesions, indicating that luminal narrowing was not the sole predictor of cerebrovascular events.36⇓–38
These studies introduced the following new concepts: 1) The degree of carotid stenosis is a weak indicator of the volume and extension of carotid plaque42⇓–44; 2) a set of plaque features identifiable by imaging are closely linked to the development of ischemic symptoms; and 3) these features can significantly increase the risk of stroke regardless of the degree of stenosis.45⇓⇓–48 Thanks to advancements in the imaging techniques to specifically target the vessel wall as opposed to the vessel lumen, considerable research effort is underway to identify those plaque-related parameters that, together with the degree of stenosis, can more accurately predict the presence of vulnerable plaque and the associated risk of ischemic events.
More than 30 years ago, Imparato et al8 found that there were certain plaque features, such as IPH, that were associated with an increased risk of plaque rupture and distal embolization. Since that time, roughly 1000 articles have been published on IPH as well as ones characterizing additional features associated with plaque vulnerability, including the thickness of the fibrous cap, rupture of the cap, the presence and size of the LRNC, and the presence of active plaque inflammation. Vulnerable plaques also tend to be characterized by an eccentric distribution, an irregular surface of the intimal layer, or superficial ulcerations with intimal exposure.
Imaging Features of Plaques at Risk for Stroke
US can assess plaque composition based on echogenicity with classification systems proposed by Geroulakos et al49 and Bluth.50 The presence of echogenic/hypoechogenic elements is associated with the LRNC,51,52 whereas hyperechogenic regions or the presence of acoustic shadowing is indicative of calcification. US is sensitive in identifying calcification, but when present, the ensuing acoustic shadowing limits visualization of tissues deep to the calcification.53
CT has been used to type plaques based on Hounsfield attenuation. de Weert et al54,55 categorized plaques as fatty for attenuation values of <60 HU, mixed for attenuation values between 60 and 130 HU, and calcified for attenuation values of >130 HU. By applying these thresholds, it is possible to identify those plaques with an LRNC from others with a prevalent expression of myofibroblasts, hemorrhage, or calcification.56 Based on this analysis, calcified plaques were found to be 21 times less likely to be symptomatic than noncalcified plaques,57 whereas fatty plaques were clearly associated with an increased risk of rupture.48,58
MR imaging has the ability to distinguish plaque components such as the LRNC, fibrous tissue, and IPH with high accuracy.59⇓⇓⇓⇓–64 The identification of calcified components can be more challenging than CT, but MR imaging typically offers good results.65,66
Luminal Morphology and Ulcerations
The morphology of the luminal surface of carotid plaques can be classified as smooth, irregular, or ulcerated.67 A smooth surface is identified as plain luminal morphology without any sign of ulceration or irregularity. An irregular surface indicates the presence of small alterations of the luminal surface on the luminal profile of the plaque; this condition is considered a risk factor for embolism and is associated with an increased risk of TIA/stroke.61 The third type of morphology is ulceration. Plaque ulceration has been defined as “an intimal defect larger than 1 mm in width, exposing the necrotic core of the atheromatous plaque”68; however, other authors suggested other (smaller) sizes.69⇓–71 The NASCET study demonstrated a significantly increased risk of cerebrovascular events in plaques with ulcerations.4
Intraplaque Hemorrhage
IPH is a common feature of atherosclerotic plaques and is considered one of the identifying features of vulnerable plaque.40 A number of studies have found a statistically significant association between the presence of IPH and cerebrovascular events,72,73 and IPH has been implicated in plaque progression.74 It is thought that the rupture of neovessels or plaque rupture itself causes IPH; and some conditions such as inflammation, metabolic disease, or diabetes may precipitate this event.75 Recent literature also indicates a potential role of blood pressure.76,77
Fissured Fibrous Cap and Lipid Rich Necrotic Core
The fibrous cap (FC), which separates LRNC from the vessel lumen, is considered one of the most important features of the carotid artery vulnerable plaque model. The FC is a layer of fibrous connective tissue and contains macrophages and smooth-muscle cells within a collagen-proteoglycan matrix associated with T-lymphocytes.78 Vulnerable plaques are characterized by the presence of a thin FC covering a large LRNC containing macrophages and inflammatory cells. In both cross-sectional and longitudinal studies,21,47 the LRNC size was found to be a strong predictor of fibrous cap disruption. The fissuring or rupture of the FC exposes the LRNC to luminal blood, activating the thromboembolic cascade. Therefore, LRNC and FC status are expected to be associated with a risk of cerebrovascular events, as shown in a single-center experience.73,79,80 The intact thick FC is associated with a low risk of plaque rupture, a thin FC is associated with a mild risk, while a fissured FC is associated with a high risk of plaque rupture.81 Additionally, percent LRNC area exceeding 40% of vessel wall area indicates a high risk for plaque rupture, while percent luminal stenosis did not correlate with plaque rupture.21
Neovascularization and Inflammation
Intraplaque neovascularization arises from newly formed microvessels that grow into the intima through breaks in the medial wall and are characterized by leaky capillaries with an endothelial lining that is immature and imperfect due to the harsh atherosclerotic environment. Histopathologic studies have demonstrated that neovessels can be found within carotid artery plaques, and the degree of neovascularity is associated with the “activity” of the plaque in terms of inflammation and increased risk of neovessel rupture and hemorrhage (IPH).82,83 Inflammation of unstable “vulnerable” atherosclerotic plaques was first identified in coronary artery lesions and subsequently demonstrated in carotid artery plaques.84,85
The recruitment of inflammatory cells in atherosclerotic lesions is a constitutive phenomenon seen throughout the process of lesion initiation and plaque growth. In addition, inflammation appears to play a role in the process of plaque disruption.86 Inflammatory cells are typically found in the plaque shoulder, cap, or both.85 In many instances and particularly in advanced plaques with a complex architecture, inflammatory cells tend to accumulate focally within plaques.85,86
Several types of inflammatory cells are detected in the carotid artery vulnerable plaque, and some studies have found that the presence of macrophages is significantly associated with the risk of plaque rupture87⇓–89; therefore, the identification of macrophages has become the target of imaging studies devoted to the detection of plaque inflammation.90⇓⇓⇓–94
Plaque Remodeling (Positive versus Negative)
The concept of plaque remodeling was initially described for atherosclerotic lesions in coronary arteries but is largely accepted for other vascular beds, including the carotid arteries.95,96 Carotid plaques can show either positive or negative remodeling or both. Positive remodeling is dilation of the vessel wall in response to an increase in plaque volume with little or no compromise of the vessel lumen as the vessel initially attempts to maintain normal lumen diameter.97 Negative remodeling is present when the vessel lumen diameter is decreased (stenosis).
Plaque Volume
Recent studies have demonstrated that the volume of the carotid artery plaque could play a role in determining plaque “vulnerability” and risk of cardiovascular events.98,99 Increasing plaque volume predicted cardiovascular events.43,100 Some authors have hypothesized that the plaque volume may be a better indicator of the severity of atherosclerotic disease than the degree of stenosis.21 Noninvasive in vivo assessment of atherosclerotic plaque volume and the relative contribution of different plaque components clearly have important clinical implications as they relate to risk assessment for ischemic events. In addition, it has been shown that higher LRNC volumes appear to be associated with the presence of plaque ulcerations, representing a significant risk factor for the development of cerebrovascular events.101 Furthermore, plaque composition is known to change with increasing plaque volume. More specifically, there is an increase in the proportion of lipid and calcification with increasing plaque volume.101 Plaque length, which relates more directly with plaque volume than the degree of stenosis, has been shown to be an independent risk factor for periprocedural complications and an excess restenosis rate in a secondary data analysis of the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS).102
Summary Concepts
It is clear that there are several plaque features of increased clinical risk supported by associations with endarterectomy specimen analyses. It is of utmost importance to test management strategies based on these MR imaging–defined features of risk before treatment guidelines can be established. Currently, there are several prospective trials intended to examine the value of prospective plaque imaging (Atherosclerosis Risk in Communities [ARIC], Plaque At RISK [PARISK], Carotid Plaque Imaging in Acute Stroke [CAPIAS], Chinese Atherosclerosis Risk Evaluation-Phase II [CARE-II], and Canadian Atherosclerosis Imaging Network [CAIN]).103⇓⇓⇓⇓–108 In the meantime, it is possible that carotid plaque characterization may be of immediate clinical value today. Given that the presence of IPH, large LRNC, and/or a thin-ruptured FC is associated with a higher risk of future cardiovascular events, the presence of these plaque features may warrant closer clinical follow-up and consideration for more intensive medical therapy. Despite attempts to encourage all physicians to manage atherosclerosis medically with current evidence-based guidelines, many patients are not receiving high-intensity lipid-lowering therapy, even when indicated. Providing additional information based on carotid plaque MR imaging identification of IPH, large LRNC, and/or a thin-ruptured FC may improve patient/physician compliance with current therapeutic guidelines. If patients receiving standard-of-care medical therapy have repeat strokes ipsilateral to carotid plaque harboring “vulnerable” plaque features, they may warrant surgical intervention even if they do not meet the stenosis thresholds by the NASCET criteria.
Current Imaging State of the Art
In this section of the paper, we will summarize the imaging techniques that can be used in the imaging of the carotid artery wall.
US
US is generally accepted as the standard imaging technique for first-line diagnosis of atherosclerosis of the carotid artery.67 US has shown very good results in the identification and characterization of high-risk plaques in patients with atherosclerosis.109 In particular, the use of microbubble contrast material facilitates assessment of vulnerable plaque features such as the presence/absence of neovascularization.110,111 The recently introduced volumetric US technology seems to add further value to this technique by improving the interobserver concordance and increasing the spatial coverage.112 Another US technique that could be used for carotid plaque characterization is intravascular US (IVUS). The advantage of IVUS is excellent spatial resolution, which is possible given the short distance between the probe and the carotid plaque, which permits the use of high-frequency (up to 50 MHz) insonation without excessive attenuation.
US, however, suffers from some key limitations. In patients with short muscular necks, it may be very difficult to identify the carotid bifurcation.113 In obese patients or in patients that have had radiation therapy, US assessment of the carotid arteries can be challenging. Another limitation of US is the evaluation of highly calcified plaques that create acoustic shadowing that can reduce visualization of the lesion.114 Furthermore, US is less capable of detecting additional, more distally located (“tandem”) stenoses than CTA or MRA. It is important to underline that IVUS is invasive and is only performed in selected cases that are largely treated with carotid artery stent placement; thus, no pathologic correlate is available. IVUS identification of the fibrous cap or visualization of friable plaque may correlate with increased risk of emboli. Moreover, the small cohorts of assessed IVUS patients as well as the potential risk related to the procedure need further analysis before IVUS can be included in the routine clinical work-up.115
Luminal Morphology and Ulcerations.
It has recently been shown that 3D-US could be effective in the detection of ulceration in carotid artery plaques.116,117
Intraplaque Hemorrhage.
A few articles have assessed US performance in the detection of IPH, and the results demonstrated low sensitivity and specificity.118,119
Fibrous Cap Status.
Some authors have explored the potential of conventional US to characterize the FC, but the results obtained were suboptimal.120⇓–122 Recent articles123,124 have suggested that intravascular ultrasonography can assess in detail plaque structure and the FC but with associated procedural risk.
Neovascularization and Inflammation.
Several recently published110,111,125,126 contrast-enhanced sonography studies found that sonographic enhancement correlates with intraplaque neovascularization in carotid endarterectomy specimens. However, the reproducibility and utility of this technique for clinical care are not well-established.127
It is important to underline that US can also be used to assess those initial subtle wall alterations in the very early phases of atherosclerosis progression, for example, the intima-media thickness that is considered a significant predictor of coronary and cerebrovascular events.128,129
Recent studies assessed the reliability of US for assessing certain plaque features. Bar et al130 assessed plaques in 30 patients. Interrater agreement values for the following plaque features were as follows: homogeneity, 96% (κ = 0.84; P < .001); surface characteristics, 90% (κ = 0.77; P < 001); and echogenicity, 86% (κ = 0.60; P < .001). The correlation coefficient for plaque content and volume measurement agreement was 0.81, and measurements did not differ significantly (P = not significant). In an article published in 1999 by Hartmann et al,131 the κ values and 95% confidence intervals for interrater reproducibility were 0.05 (−0.07 to 0.16) for plaque surface structure, 0.15 (0.02 to 0.28) for plaque heterogeneity, 0.18 (0.09 to 0.29) for plaque echogenicity, and 0.29 (0.19 to 0.39) for plaque calcification. The upper bounds of all the confidence intervals were below the 0.40 level, indicating very low reliability.
CT
Modern CT scanners can provide exquisite, rapid high-resolution imaging of the carotid artery lumen and the arterial wall. The introduction of multienergy technology provided a tremendous boost to the development of CT techniques; and constant advances in detector technology, in spatial and temporal resolution, and release of advanced software for image reconstruction have helped to consolidate this technique as a reliable tool for the evaluation of arterial pathology, with particular success in the detection and characterization of carotid atherosclerosis.
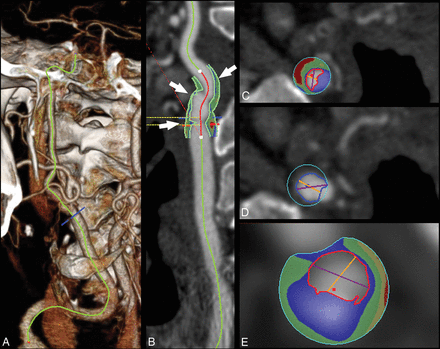
Because of its spatial resolution, CT imaging seems to be a promising tool for the quantification of the volume of the carotid plaque as well as for the volume quantification of plaque subcomponents (fatty, mixed, calcified) (Fig 1). Moreover, the introduction of multienergy technology is opening new options in tissue characterization because the different tissue components show different attenuation levels with varying kiloelectron volt values.132,133
Plaque volume analysis in a 75-year-old man with a TIA. In the volume-rendered image, the carotid is traced (A), and in the curved-planar-reconstructed postprocessed image (CTA module, Aquarius iNtuition Edition, Version 44121382907; TeraRecon, San Mateo, California) (B), the plaque is identified based on the green contours (white arrows). The volume analysis with automated boundary detection and tissue segmentation is shown in panels C, D, and E (corresponding to the 3 arrows, proximal-to-distal) with contours delineating the lumen (red contour), outer wall (blue contour), and shading of calcium (blue), mixed tissue (green), and lipid component (red).
Luminal Morphology and Ulcerations.
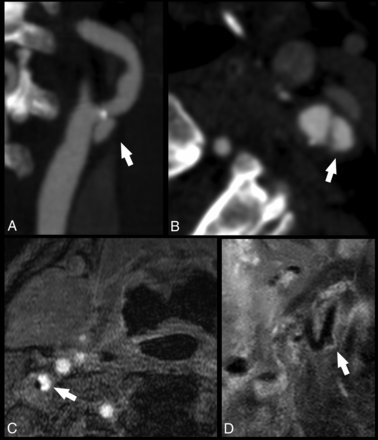
CT offers very good results in detecting ulcerations when compared with histopathology (Fig 2), with performance significantly better than US,134⇓–136 but the presence of a halo or edge blur may hinder detection of smaller ulcerations.
Ulcerated carotid artery plaques detected with CT and MR imaging. In the first case, the CTA of a 74-year-old man with a TIA demonstrates an ulcerated carotid artery plaque (white arrows) in the left internal carotid artery (white arrow) in the MIP (A) and axial source (B) images. In the second case, an MR imaging analysis of a 63-year-old man with a TIA shows a tiny ulceration (white arrows) in the right internal carotid artery visible in the axial (C) and paracoronal (D) planes.
Intraplaque Hemorrhage.
Detection of IPH using CT is challenging, and conflicting results have been reported. While some authors have found that CT density is slightly higher in fatty plaques with IPH identified by MR imaging compared with plaques without IPH,137 others found no significant differences in Hounsfield units of fatty plaques with and without IPH identified by MR imaging.104 Other authors found a correlation between the presence of IPH and low Hounsfield unit values (<30 HU),56,138,139 which might be explained by the associated presence of LRNC. Recently, some authors suggested that the rim sign on CTA (soft-tissue plaque with adventitial calcifications) as well as maximum soft-plaque thickness could be predictive of carotid IPH.140
Fibrous Cap Status.
The assessment of the status of the FC using CT is complex because of the artifacts related to the edge blur and halo effects, but authors suggest that CT can be used to assess the FC status, in particular to identify rupture.141,142 Notably, it seems that the rupture of the FC correlates with the presence of postcontrast plaque enhancement in CTA analysis.45
Neovascularization and Inflammation.
The degree of postcontrast plaque enhancement has been shown to be associated with the extent of neovascularization on CT.143 The adventitial neovascularization has been assessed with both MR imaging144 and CT.145,146 Romero et al145 showed adventitial neovascularity on CTA to be significantly more common in symptomatic than in asymptomatic patients with ≥70% stenosis and for stenosis between 50% and 70%,146 and similar findings have been reported by MR imaging.144
Plaque Remodeling.
CT studies have found that positive carotid remodeling was significantly greater in patients with cerebral ischemic symptoms than in asymptomatic patients and that the extent of expansive remodeling may indicate underlying atherosclerotic plaque vulnerability.147,148
Plaque Volume.
CT can calculate the volume of the carotid artery plaque and determine the volume of the subcomponents, according to the Hounsfield unit threshold.149 Further, it has been shown in a CT/MR imaging comparison study that the best discriminating factor for predicting a complicated American Heart Association type VI plaque is the thickness of the fatty plaque component with a receiver operating characteristic area under the curve of 0.89.104
CT Limitations.
CT imaging has 3 main limitations: 1) the radiation dose delivered to patients, 2) the risk related to the administration of contrast material, and 3) the limited fatty tissue contrast. Diagnostic radiation exposure and the consequent potential radiation hazards represent a significant issue,150,151 particularly when longitudinal monitoring is required. The second limiting factor of CT is the potential anaphylactic reaction to contrast material152,153 and contrast-induced nephropathy, which is a common form of hospital-acquired acute renal failure.154,155 A further limitation of CT is the limited published information concerning the reliability of this technique and the prospective value of CT-based plaque features on stroke risk and/or stroke recurrence.
MR
The use of a high field strength (1.5T–3T) and dedicated surface radiofrequency coils improves the signal-to-noise ratio, which allows for the evaluation of plaque components and investigation beyond the simple assessment of stenosis measurements. Multicontrast carotid MR imaging (including T1WI, T2WI, and proton density and TOF) can characterize plaque components (eg, fibrous cap, LRNC, calcification, and intraplaque hemorrhage) without administration of contrast agents. Contrast-enhanced MR imaging improves tissue characterization60,61 and offers information on the presence of neovascularization (Figs 3 and 4).
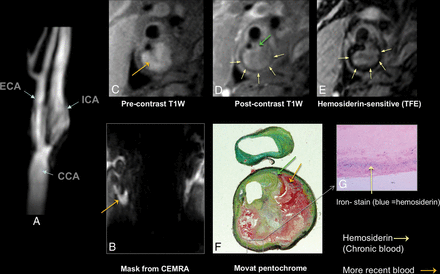
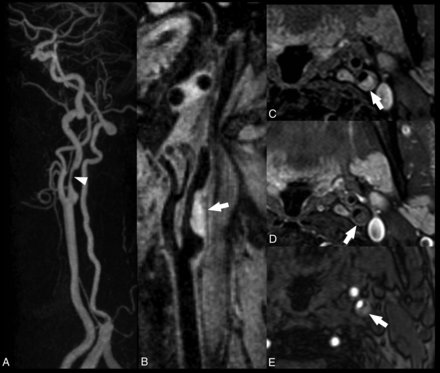
Carotid atherosclerotic plaque MR imaging and a specimen from a 73-year-old man with stenosis of the carotid bulb measuring 69% by the NASCET criteria demonstrated on a contrast-enhanced MRA (A). The precontrast (mask) image from the contrast-enhanced MRA demonstrates bright signal indicative of intraplaque hemorrhage, specifically subacute blood, or methemoglobin (B, arrow). Subacute blood is also identified as bright signal on the precontrast T1-weighted black-blood image (C, arrow). A rim of hemosiderin is identified as hypointense signal on the postcontrast black-blood image (D) and a hemosiderin-sensitive sequence (E) and is confirmed on the endarterectomy specimen (F and G). The fibrous cap is also delineated (green arrow, D and F). Black-blood imaging was achieved by using 2D cardiac-gated double inversion recovery turbo spin-echo. ECA indicates external carotid artery; CCA, common carotid artery; TFE, turbo field echo.
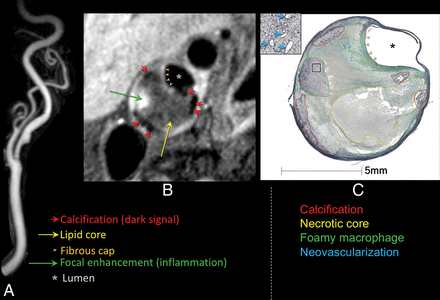
Carotid atherosclerotic plaque MR imaging and a specimen from a 76-year-old woman with transient ischemic attacks ipsilateral to carotid bulb stenosis, measuring 47% by the NASCET criteria demonstrated on a contrast-enhanced MRA. A, Narrowing is caused by the plaque characterized by 2D cardiac-gated double inversion recovery black-blood MR imaging (B). Regional enhancement (green arrow) within the lipid core (yellow arrow) suggests focal inflammation with neovascularity as confirmed on the endarterectomy specimen (C, green circle). Contrast enhancement is also useful for delineating the fibrous cap (B and C, orange arrowheads). Calcification is identified as areas of hypointensity (B, red arrows, and C, red circle).
Luminal Morphology and Ulcerations.
MR imaging detects plaque ulcerations with a sensitivity similar to CT (Fig 2).156 Etesami et al156 demonstrated that the use of contrast-enhanced MRA techniques improved the sensitivity for ulcerations by 37.5% compared with an unenhanced time-of-flight sequence.
Intraplaque Hemorrhage.
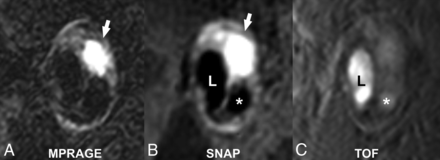
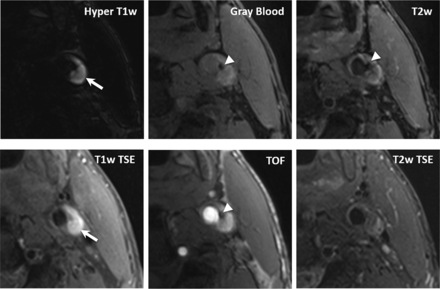
MR imaging is considered the best imaging technique for the detection of IPH (Figs 3 and 5). Several studies have shown that the appearance of IPH depends on the oxidative state of hemoglobin.157⇓–159 Because of the sensitivity of MR imaging in detecting IPH and the risk attributed to this feature, some authors suggest that MR imaging is the best technique for imaging carotid artery vulnerable plaque.12,40,63,160,161 During the subacute and chronic phases, IPH appears bright on T1-weighted imaging due to the relatively short T1 of methemoglobin. This phenomenon has been exploited using widely available sequences such as MPRAGE, though other heavily T1-weighted techniques have been developed to satisfy this purpose such as multicontrast atherosclerosis characterization (MATCH) and simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) (Figs 6 and 7). In MATCH, hyper-T1 contrast weighting is achieved using inversion preparation and data acquisition at the background nulling point, and thus IPH can be exclusively visualized with a near-dark background; on the other hand, background tissues can readily be visualized on other contrast weightings, thanks to the inherently coregistered multicontrast acquisition (Fig 7).162,163 IPH detection using 3D SNAP enables greater conspicuity of the lumen boundary compared with MPRAGE (Figs 5⇓–7).164 SNAP provides the advantage of 3D isotropic resolution as well as simultaneous bright-blood angiography to detect stenosis or ulceration that may be colocalized with IPH.165 It is worth noting that it is not necessary to restrict carotid wall imaging to dedicated, small-FOV surface coils for IPH detection since this can be achieved at a lower spatial resolution using large-FOV neck coils (Fig 3).63,79,166 In fact, IPH detection can be achieved on the mask sequence acquired as part of a routine contrast-enhanced MRA (Fig 3).63
Smooth left internal carotid artery stenosis with intraplaque hemorrhage. All images were acquired with a 16-channel neurovascular coil at 3T. The CE-MRA demonstrates a smooth, nonulcerated stenosis in the bulbous and postbulbous parts of the left internal carotid artery (white arrowhead, A). Oblique reformat of a coronally acquired MPRAGE image shows extensive intraplaque hemorrhage, which appears hyperintense (white arrow, B). The IPH is hyperintense on the nonenhanced T1 fat-saturated spin-echo image (C) and isointense on the gadolinium-enhanced T1 fat-saturated spin-echo image (D). On the TOF MRA source data, the IPH also appears hyperintense but to a lesser degree than the intraluminal flow signal (E).
Matched cross-sectional images of a carotid plaque with high signal intensity (white arrows), consistent with the presence of intraplaque hemorrhage on MPRAGE (A) and SNAP MR imaging (B). Note the greater conspicuity of the carotid lumen (L) on SNAP compared with the MPRAGE image. There is a penetrating ulcer (asterisk) that is more easily detected on SNAP compared with the TOF MRA image (C).
In a 68-year-old male patient, coexistent plaque components, fresh intraplaque hemorrhage (arrows), and superficial calcifications (arrowheads) are detected by MATCH (first row) and the conventional multicontrast protocol (second row). Compared with T1-weighted TSE and TOF, MATCH provides more conspicuous depiction of intraplque hemorrhage on the hyper-T1-weighted image and calcification on the gray blood image. Notice that the calcification is also visible on the MATCH T2-weighted image but not on the T2-weighted TSE image.
Lipid-Rich Necrotic Core.
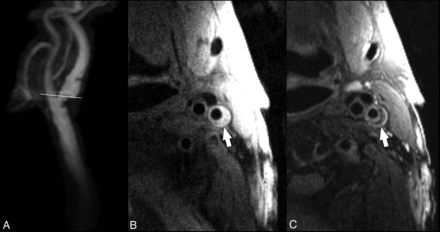
Initial research demonstrated that LRNC could be detected as a focal hypointense region on T2WI (Fig 8).167,168 Multiple studies have confirmed the improved detection of LRNC seen as a focal nonenhancing region on contrast-enhanced T1WI within the carotid vessel wall.60,61 Larger LRNC size correlates with future ipsilateral carotid symptoms. All validation of LRNC detection/quantification and predictive features has been based on multicontrast carotid plaque MR imaging protocol using dedicated carotid coils. Recent work has suggested the ability to detect a large LRNC using commercially available 3D T1WI sequences and large-FOV neck coils. The Canadian Atherosclerosis Imaging Network has recently completed a prospective, multi-institution study using large-FOV neck coils and commercial sequences from a variety of MR imaging vendors to detect LRNC and IPH. When fully analyzed, CAIN may give us additional information about the ability of IPH and LRNC size detected with large-FOV neck coils and commercial sequences to predict future ipsilateral events.
A, Contrast-enhanced MRA of the extracranial carotid bifurcation indicating the level of 2D-FSE images obtained with 1.5T. B, T1-weighted double inversion recovery black-blood FSE image shows an eccentric plaque (arrow) in the internal carotid artery. C, T2-weighted double inversion recovery black-blood FSE image at the same level shows a crescentic, hypointense signal from the necrotic core, which is separated by a higher intensity fibrous cap from the flow lumen.
Fibrous Cap Status.
MR imaging can assess fibrous cap status,61,169 as opposed to the other noninvasive imaging modalities such as CT and US.141 A regular (thick) FC is characterized by the presence of a juxtaluminal band of low signal on time-of-flight MR images and/or a hyperintense juxtaluminal region on contrast-enhanced T1WI, whereas a thin FC is present when this band of low signal on TOF or the hyperintense region on contrast-enhanced (CE)-T1WI is not visible or when the juxtaluminal hyperintense region on CE-T1-weighted MR imaging is interrupted. The fissured fibrous cap is characterized by 2 distinct features: 1) the absence of the juxtaluminal band of low signal, and 2) the presence of a bright gray region adjacent to the lumen, corresponding to plaque hemorrhage and/or mural thrombus.60,170 As shown by Wasserman et al61 and Cai et al,60 contrast-enhanced imaging could be useful for improving delineation of the cap compared with noncontrast (T2), and CE-T1WI can be used to quantify the fibrous cap and the LRNC. Although resolving a thin fibrous cap defined by pathologic criteria would necessitate a higher field strength to overcome signal constraints, distinguishing thin/ruptured from thick fibrous cap thicknesses can be achieved at 1.5T (Fig 8).171,172
Consistent visualization of the FC requires dedicated carotid surface coils. Yuan et al170 and others have shown that the fissured FC has a statistically significant association with the presence of cerebrovascular symptoms81 and is associated with a higher risk of ischemic symptoms in prospective studies.79,80
Neovascularization and Inflammation.
There are new contrast agents using iron particles (ultrasmall superparamagnetic iron oxide or P947)173,174 that can evaluate plaque inflammation via uptake by phagocytic cells within the inflamed vessel wall. Small particle–based MR imaging contrast agents (iron oxide) can be used to evaluate the presence of plaque inflammation. These iron oxide particles enter atherosclerotic plaques, with the agents accumulating in macrophages transformed from blood monocytes attracted by inflammatory mediators.90 High-risk inflamed plaques contain a focal area of signal loss on MR images, due to iron oxide accumulation.91 Iron nanoparticles (10- to 300-nm-sized) are also bound to antibodies, drugs, peptides, and polysaccharides; and avidin-biotin cross-linked with polymers is used to assess endothelial function in animal models. Polymer hydroxyl acidic core (polylactic acid) and dendrimers (polyamidoamine, diaminobutane) have been described as suitable to functionalize the surface of superparamagnetic iron oxide175 (15- to 60-nm superparamagnetic iron oxide) particles, allowing for ligand binding. Ligand-bound superparamagnetic iron oxide (anti-VCAM-1 and anti-E-selectin antibody conjugated superparamagnetic iron oxide) can cause dephasing and loss of T2* signal intensity due to susceptibility effects and is suitable for passive targeted imaging of inflammation in cardiovascular tissue.175 In addition to iron oxide nanoparticles, various other nanoparticles are being used for molecular imaging of atherosclerosis in animal models, eg, liposome vesicles (50–70 nm) for US176/MR imaging177; perfluorocarbon core emulsions (200–300 nm) for MR imaging, US, fluorescence, and nuclear imaging; chemo-thermo-immuno178; and high-density lipoprotein and low-density lipoprotein micelles for MR imaging.179 Other types of particles such as gold, carbon nanotube fullerenes (4 nm), quantum dots cadmium selenide spheres (2–10 nm), and metal-based agents are in the process of standardization and may be useful in fluorescent imaging.180 Moreover, other investigators have reported the possibility of viral capsid protein cages with gadolinium as potential nanospheres for drug encapsulation and imaging.181
A recent MR imaging study182 showed that enhancement of carotid plaque after administration of gadolinium is associated with neovascularization (P < .001) (Fig 4). The correlation between the degree of plaque enhancement and the degree of neovascularization, which is itself linked to the degree of plaque inflammation, was also confirmed at histology in a recent study by Millon et al.182 Investigations have shown that inflammatory cells are also present at the interface with the underlying necrotic core and in the plaque shoulder region.183,184 From the imaging point of view, it is possible to distinguish 2 different types of neovascularization: 1) adventitial neovascularization, and 2) intraplaque neovascularization. The adventitial neovascularization has been assessed with MR imaging.144 Ectopic neovascularization in the intima and media is a hallmark of advanced atherosclerotic lesions, but the adventitial layer is a fundamental target because it serves as the main source of in-growth of new vessels. The degree of neovascularity measured using gadolinium perfusion methods correlated with adventitial perfusion as measured by its transfer constant (Fig 9).185 Wasserman171 categorized the circumferential enhancement (0, absent; 1, <50%; 2, ≥50%) on postcontrast MR imaging by finding an association between the grade of adventitial enhancement and cerebrovascular events. Plaque perfusion imaging using dynamic contrast-enhanced MR imaging has been shown to give reproducible physiologic measurements of the vasa vasorum.186,187 However, protocol compliance may be more important for functional imaging such as dynamic contrast-enhanced MR imaging as compared with anatomic imaging.
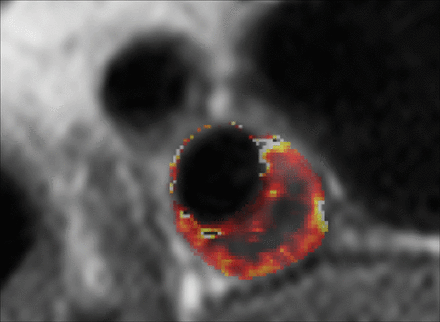
Volume transfer constant (Ktrans) map of a patient with carotid plaque. Maps were generated using pharmacokinetic modeling of dynamic contrast-enhanced MR images. The parametric map is overlaid on the anatomic MR image, and voxel Ktrans values (Patlak model) are color-coded. The necrotic core exhibits low Ktrans values at the center of the plaque, while the highly vascularized adventitia at the outer rim exhibits high Ktrans values. There is another region of higher Ktrans values near the inner rim of the plaque.
Plaque Volume.
Recently published studies show the utility of MR imaging for this type of quantification.103,188 In general, the reliability of MR imaging for plaque assessment has been very good. A study published by Wasserman in 2010103 found that the scan reliability for common carotid artery lumen area was 0.94, whereas for the ICA lumen area, it was 0.89. In the assessment of the total wall volume, the value was 0.79, but in the assessment of LRNC volume, the value was very low (0.3). The authors found that overall reliability is primarily related to reader variability rather than scan acquisition. The coefficient of variation values for the plaque area or plaque volume are between 3% and 6%, as demonstrated by Saam et al.189,190
Imaging studies have documented changes in atherosclerotic plaque volume and composition and progression of subclinical lesions into rupture-prone plaques.191⇓⇓⇓⇓–196 The ability to monitor these changes might contribute to our ability to estimate risk and assess pharmaceutical treatment efficacy.197 For example, changes in plaque structure that correspond with a clinical event help to identify that plaque as a culprit lesion, which puts it at a higher risk for future stroke.34,122,198 Several studies have reported using MR imaging for longitudinal analysis of carotid plaque variations,191⇓–193 with fewer reports using CTA.194,195
MR Imaging Limitations.
An important limitation to contrast-enhanced MR imaging evaluation of plaque that has recently emerged is the potential for gadolinium toxicity, particularly when longitudinal monitoring is required. Recent studies have reported the accumulation of gadolinium in various tissues of patients without renal impairment, including in bone, brain, and kidneys,199⇓–201 and in July 2015, the US Food and Drug Administration published a safety announcement that it is investigating the risk of brain deposits associated with the repeat use of gadolinium contrast agents in MR imaging,202 stating: “To reduce the potential for gadolinium accumulation, health care professionals should consider limiting GBCA [gadolinium-based contrast agents] use to clinical circumstances in which the additional information provided by the contrast is necessary. Health care professionals are also urged to reassess the necessity of repetitive GBCA MRIs in established treatment protocols.” This risk must now be weighed against the potential radiation hazard described earlier that limits longitudinal plaque monitoring by CT.
Advanced Algorithms to Carotid Artery Plaque Characterization
With the development of deep learning technology and plaque characterization algorithms applied to medical imaging, it is now possible to identify, classify, and quantify target features from imaging datasets such as total carotid artery plaque volume and plaque subcomponent detection (calcium, IPH, lipid core).203,204 Deep-learning technology has experienced rapid progress in health care over recent years, with early reports of implementation in carotid imaging204 raising the prospect of routine use in the clinical setting once validated.
Functional–Molecular Imaging
“Molecular” imaging techniques have been gaining popularity. The objective of molecular imaging is to provide biologic insight into the identification and classification of carotid artery plaques, especially those at high risk. In atherosclerotic plaques, multiple and complex reactions take place at the molecular and cellular level, with various atherosclerosis-related biomarkers present at different stages of disease progression.205 Conventional imaging with US, MR, or CT cannot identify these components because of limited imaging contrast; therefore, several methods have been proposed that use external contrast agents targeting these specific biomarkers.
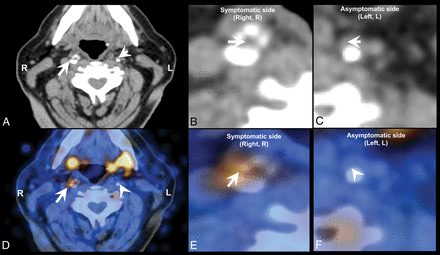
A wide variety of studies has assessed the diagnostic potential of nuclear medicine techniques for imaging and quantifying plaque inflammation, such as by PET using the widely available [18F] FDG or newer radiotracers such as [18F] fluorocholine (Fig 10).206⇓–208 Nuclear medicine tracer techniques have also shown efficacy in the identification of neovascularization.209,210
[18F] fluorocholine positron-emission tomography CT (18F-FCH PET CT) image of a symptomatic (arrow) and contralateral asymptomatic (arrowhead) carotid plaque of a patient who experienced right-sided stroke. A, Diagnostic contrast-enhanced CT shows a significant stenosis in the right internal carotid artery because of a calcified plaque, whereas a noncalcified atherosclerotic plaque can be seen on the contralateral internal carotid artery. B, CT, inset on the symptomatic plaque. C, CT, inset on the asymptomatic plaque. D, The fused PET CT image denotes a focal area of high [18F] FCH uptake in the right symptomatic carotid plaque, whereas there is no visible [18F] FCH uptake in the left asymptomatic carotid plaque. E, Fused PET CT, inset on the symptomatic plaque. F, Fused PET-CT, inset on the asymptomatic plaque.
Because vulnerable plaques are infiltrated by lymphocytes and macrophages, with the latter cell population capable of taking up [18F] FDG from the interstitial spaces, [18F] FDG PET can be used to directly detect plaque inflammation in various anatomic locations.211 In recent years, a number of studies have assessed the diagnostic potential of [18F] FDG PET to image and quantify plaque inflammation206,207 as well as monitoring the reduction of plaque inflammation resulting from statin therapy.208
Recommendations
Carotid MRI
Background.
Results from recently published meta-analyses support the hypothesis that MR imaging detection of carotid IPH is associated with increased risk for future primary and recurrent ischemic neurologic events.79,212,213 Furthermore, the absence of IPH portends a benign clinical course, even among patients with symptomatic 50%–99% carotid stenosis.213 Other plaque features associated with increased risk include identification of a large LRNC and a thin or ruptured fibrous cap.79
Goals.
1) To provide general guidelines for carotid MR vessel wall imaging with recommended imaging sequences, spatial resolution, and coverage. Guideline considerations are that the protocol can be applied broadly across a spectrum of clinical scanners and not require specialized software or research keys for implementation, and 2) to recommend future areas for technical development and clinical expansion needs.
Essential Features for Identification with Carotid Plaque Imaging.
Any MR imaging protocol for plaque imaging should be able to identify the following atherosclerotic plaque characteristics:
Stenosis and luminal surface condition (fibrous cap and ulceration).
Presence of intraplaque hemorrhage.
Presence of lipid rich necrotic core.
Plaque burden and distribution.
Minimum MR Imaging Protocol Requirements for Identification of Essential Plaque Features (1.5T and 3T).
Recommended minimum sequence requirements are the following:
Resolution: In-plane 0.6 mm, through-plane 2 mm
Longitudinal coverage: 3–4 cm centered on the carotid bifurcation
Effective blood suppression for a plaque burden visualization sequence.
The protocol may include any combination of sequences that meets the minimum requirements set forth above. The sequences used can be either 2D or 3D or a combination, provided that they together meet the minimum sequence requirements above. Overall scan time can be adjusted based on field strength and the availability of specialized hardware such as carotid phased array coils. 3T scanners are recommended for improved SNR.
Example Protocols.
Four protocols are presented based on considerations for 2D and 3D imaging and the use of gadolinium contrast agents (Tables 1⇓⇓–4). If patients are able to undergo gadolinium contrast injection, its use is recommended for the detection and quantification of LRNC and the delineation of the fibrous cap.60,214 Use of large-coverage 3D sequences can detect plaques extending beyond the 4-cm coverage centered on the bifurcation and is preferable. Carotid coils are recommended for use with all protocols, though large-FOV neck coils can detect IPH. It is possible to add a 4-minute 3D MPRAGE sequence to routine clinical carotid MRA protocol. The protocol is similar to Table 1, but with 0.8-mm isotropic resolution using a large-FOV neck coil instead of 0.6 mm using dedicated carotid surface coils. Focal regions of T1 hyperintensity within the carotid plaque that are 15× greater than the adjacent sternocleidomastoid muscle can be used to identify IPH.
3D Noncontrast protocol
3D contrast protocol
2D noncontrast protocol
2D contrast protocol
Motion-sensitized driven-equilibrium215/flow-sensitive dephasing216 flow suppression is required for 3D sampling perfection with application-optimized contrasts using different flip angle evolution (SPACE; Siemens, Erlangen, Germany)/Cube (GE Healthcare, Milwaukee, Wisconsin)/volume isotropic turbo spin-echo acquisition (VISTA; Philips Healthcare, Best, the Netherlands) to ensure effective blood suppression to accurately identify plaque lumen boundaries. Good blood suppression postcontrast requires the use of motion-sensitized driven-equilibrium or double inversion recovery (DIR)/quadruple inversion recovery217 flow suppression. For DIR, the inversion time can be calculated based on estimated T1 values of blood at 5-minute intervals following contrast administration (0.1 mmol/kg) for 1.5T or 3T scanners.218 We recommend a TI of 250 ms for 3T scanners for a TR triggered at a 1 RR interval, which generally produces adequate flow suppression beginning 5 minutes after injection despite variations in postinjection scan time and heart rate, which will affect the T1 blood values.
Discussion.
Lumen. Quantifying luminal narrowing is a prerequisite, as stenosis severity is the cornerstone for treatment decisions in current clinical guidelines. Furthermore, detection of ulceration provides prognostic value. Use of TOF MRA for lumen assessment avoids the need for IV contrast and may provide confirmatory evidence of intraplaque hemorrhage and sometimes calcification. Addition or substitution with CE-MRA should be considered for those without contraindication for contrast administration. This would also provide an opportunity to perform post-contrast-enhanced imaging of the vessel wall for direct identification of the LRNC and identification/confirmation of fibrous cap status and ulcerations.
IPH. MR imaging techniques are available for IPH detection across scanner platforms, and the predictive value of IPH for ischemic events has been extensively evaluated, both with and without custom carotid coils. In a review performed by Gupta et al,79 studies were stratified by those utilizing multisequence, carotid coil–dependent protocols and those using a single sequence with standard large-FOV neck coils for IPH detection. Using either technique, IPH was associated with significantly increased risk for TIA or stroke (hazard ratio, 440; 95% CI, 210–923; and hazard ratio, 504; 95% CI, 215-1185, respectively). While IPH can be identified on T1WI sequences such as T1WI fast spin-echo, T1WI SPACE, TOF, and so forth, a highly T1-weighted sequence such as MPRAGE can provide higher sensitivity and specificity for IPH detection.219
Lipid-Rich Necrotic Core. T2-weighted imaging can be used to detect the presence of LRNC.167,168 Direct assessment of the LRNC can also be done in patients undergoing contrast administration using a postcontrast T1WI scan. CE-MRA followed by post-CE vessel wall imaging in patients without contraindication will improve detection and quantification of the LRNC and delineation of the fibrous cap.214
Plaque Burden and Distribution. Knowledge of the location and distribution of plaque assists in preprocedural planning. Time-efficient 3D large-coverage black-blood MR imaging may be better suited for this purpose.
Future Improvements and Needs for MR Imaging.
Technical developments are urgently needed in the following areas:
Improved spatial resolution both in-plane and through-plane to better characterize finer structures such as fibrous caps.
More effective blood flow suppression for large-spatial-coverage imaging acquisition and pre- and postcontrast administration.
Dedicated carotid coils that are integrated with head and neck coils for extensive coverage.
Improved techniques for identifying the lipid rich necrotic core, especially without the need for contrast application.
More effective methods to deal with motion.
A streamlined imaging protocol that is able to identify multiple imaging targets in 1 or 2 imaging sequences.
Effective image-processing tools for efficient quantitative identification of imaging targets.
Development of training programs for MR imaging specialists on image acquisition and for radiologists on vessel wall image interpretation.
Ultimately, a guideline that clearly calls for the need for carotid plaque imaging and 1 simple protocol that can meet all the needs.
Currently, there are many new techniques being developed for carotid plaque imaging. 3D-SNAP provides non-contrast-enhanced MRA and simultaneous IPH detection.164 3D spoiled gradient-recalled echo pulse sequence for hemorrhage assessment using inversion recovery and multiple echoes (3D-SHINE) provides information about the state of IPH in addition to IPH detection.220 IPH can also be identified on a precontrast mask of CE-MRA if available.63 MATCH provides comprehensive information regarding plaque composition in a single sequence.163 3D Multiple Echo Recombined Gradient Echo (3D-MERGE)221 and 3D delay alternating with nutation for tailored excitation with fast low-angle shot (3D-DASH)222 provide large-coverage blood suppression for plaque burden measurements. Diffusion-weighted imaging can detect LRNC without the use of contrast media.223 Self-gating has been used to reject data acquired during swallowing motion.224 T1-insensitive blood-suppression techniques such as quadruple inversion recovery217 provide good blood suppression for postcontrast imaging. However, these supplementary techniques require specialized equipment (3T, custom carotid coils, custom sequences) and more intensive interpreter training.
Carotid CT
Background.
Currently no meta-analyses or prospective trials have suggested that some specific CT features are associated with an increased risk for future primary and recurrent ischemic neurologic events, even if there are several prospective trials on their way or that have been published that examine the value of plaque imaging prospectively (PARISK, CAPIAS, CARE-II).104⇓–106 However, cross-sectional studies have found that some CT characteristics (Hounsfield unit attenuation, the presence of neovascularization) are associated with increased risk of cerebrovascular events.48,225
Goals.
1) To provide general guidelines for carotid CT vessel wall imaging with recommended desirable imaging techniques, tissue contrast, spatial resolution, and coverage. Guideline considerations are that the protocol can be applied broadly across a spectrum of clinical CT scanners and not require specialized software or research keys for implementation. 2) To recommend future areas for technical development and clinical expansion needs.
Essential Features for Identification with Carotid Plaque Imaging.
Any CT protocol for plaque imaging should be able to identify the following atherosclerotic plaque characteristics:
Stenosis and luminal surface condition (plaque morphology and ulceration).
Type of plaque (fatty versus mixed versus calcified).
Presence of plaque enhancement.
Plaque burden and distribution.
Minimum CT Protocol Requirements for Identification of Essential Plaque Features.
Recommended minimum parameter requirements are the following:
Resolution: isotropic voxel with 1-mm resolution
Longitudinal coverage: from the aortic arch to intracranial vessels
CT generation: third with at least 16-detector-row.
Example Protocols.
Four protocols are presented (Tables 5⇓⇓–8). No CT study of carotid arteries must be performed without the administration of contrast material. The use of a biphasic approach (unenhanced scan followed by a contrast scan) allows the assessment of the carotid plaque neovascularization. This is becoming more important but is not considered currently necessary. To reduce the radiation dose delivered to the patients, the z-length of the basal scan should cover only the carotid artery plaque bifurcation (4-cm coverage centered on the bifurcation). The dual-energy CT technique143 allows a virtual unenhanced image to assess plaque enhancement without the need for a biphasic approach.
Aquilion Visiona
Somatom Sensation 64a
ICT
LightSpeed VCTa
Discussion.
Lumen. Quantifying luminal narrowing is a prerequisite. To correctly assess the degree of stenosis, by avoiding the halo or edge blur, the correct window settings should be used.226 At the current level of technology, the status of the FC cannot be adequately explored by CT.
Type of Plaque. According to Hounsfield unit attenuation, the carotid plaque can be categorized as fatty (<60 HU), mixed (between 60 and 130 HU), and calcified (>130 HU). By applying these thresholds, it is possible to identify those plaques with a LRNC from others. Applying the Hounsfield unit classification, however, creates 2 problems that have recently come to light: 1) The Hounsfield unit value of the plaque is dependent on the level of energy applied, as demonstrated by Saba et al132 using multienergy systems, and 2) the carotid artery plaques may show contrast enhancement (by comparing the attenuation values of the basal and postcontrast scans), suggesting that the attenuation value of the plaque obtained after administration of contrast material represents 2 different parameters: the type of the plaque and the degree of neovascularization of the tissue.143,227 This is not a problem if pre- and postcontrast scans or dual-energy is applied (capable of distinguishing plaque from contrast enhancement), but this is not usually done clinically secondary to an increase in x-ray dose.
Carotid Plaque Enhancement. Assessment of plaque enhancement is limited in the case of single-phase CTA, and multiphase CTA is rarely performed outside of research studies due to radiation concerns. An unenhanced axial CT scan obtained over 4 cm centered on the carotid bifurcation, followed by CTA, would theoretically be ideal in assessing plaque enhancement but carries a greater radiation penalty. Alternatively, some authors have employed dual-energy techniques with the use of the virtual nonenhanced image to assess plaque enhancement with less radiation dose.143
Plaque Burden and Distribution. Knowledge of the location and distribution of plaque assists in preprocedural planning. Moreover, CT can calculate the volume of the carotid artery plaque and determine the volume of the subcomponents, according to the Hounsfield unit threshold.149
Future Improvements and Needs for CT.
Technical developments are urgently needed in the following areas:
Improved contrast resolution for greater discrimination of tissue types in plaque.
Improved techniques such as multienergy applications for identifying the lipid-rich necrotic core, especially without the need for contrast application.
Evidence-based guidelines that invoke the need for carotid plaque imaging, preferably using 1 simple universal protocol that can meet all needs.
Currently, most of the research on carotid artery CT is focusing on methods that 1) reduce the radiation dose delivered to the patients, and 2) improve carotid artery plaque characterization using multienergy tools that promise more accurate detection of plaque components.
Conclusions
In the last 20 years, there has been a paradigm shift in the imaging of the atherosclerotic carotid artery, from the assessment of the degree of luminal stenosis to the characterization of plaque. Several features have been identified that are potentially associated with plaque rupture, and imaging has been used to identify these features in vivo.
Researchers and clinicians now have several imaging modalities that allow in-depth exploration of carotid artery plaque and its components. Sonography should be considered as a first-line examination, at least for screening, whereas CT and MR imaging improve identification of several plaque features associated with vulnerability.
Also promising are nuclear medicine and molecular imaging techniques that can further explore assessment of plaque vulnerability, especially inflammation, but these approaches are still investigational and not part of the main diagnostic algorithm of carotid atherosclerosis. In the future, larger prospective longitudinal studies investigating these technologic advances may fully exploit the clinical potential of vessel wall imaging.
Acknowledgments
The authors would like to thank Drs Stefan Vöö and Wei Zu for their help in image selection for this article.
Footnotes
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or US Government. The identification of specific products or scientific instrumentation does not constitute endorsement or implied endorsement on the part of the authors, Department of Defense, or any component agency.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵
- 110.↵
- 111.↵
- 112.↵
- 113.↵
- 114.↵
- 115.↵
- 116.↵
- 117.↵
- 118.↵
- 119.↵
- 120.↵
- 121.↵
- 122.↵
- 123.↵
- 124.↵
- 125.↵
- 126.↵
- 127.↵
- 128.↵
- 129.↵
- 130.↵
- 131.↵
- 132.↵
- 133.↵
- 134.↵
- 135.↵
- 136.↵
- 137.↵
- 138.↵
- 139.↵
- 140.↵
- 141.↵
- 142.↵
- 143.↵
- 144.↵
- 145.↵
- 146.↵
- 147.↵
- 148.↵
- 149.↵
- 150.↵
- 151.↵
- 152.↵
- 153.↵
- 154.↵
- 155.↵
- 156.↵
- 157.↵
- 158.↵
- 159.↵
- 160.↵
- 161.↵
- 162.↵
- 163.↵
- 164.↵
- 165.↵
- 166.↵
- 167.↵
- 168.↵
- 169.↵
- 170.↵
- 171.↵
- 172.↵
- 173.↵
- 174.↵
- 175.↵
- 176.↵
- 177.↵
- 178.↵
- 179.↵
- 180.↵
- 181.↵
- 182.↵
- 183.↵
- 184.↵
- 185.↵
- 186.↵
- 187.↵
- 188.↵
- 189.↵
- 190.↵
- 191.↵
- 192.↵
- 193.↵
- 194.↵
- 195.↵
- 196.↵
- 197.↵
- 198.↵
- 199.↵
- 200.↵
- 201.↵
- 202.↵
- 203.↵
- 204.↵
- 205.↵
- 206.↵
- 207.↵
- 208.↵
- 209.↵
- 210.↵
- 211.↵
- 212.↵
- 213.↵
- 214.↵
- 215.↵
- 216.↵
- 217.↵
- 218.↵
- 219.↵
- 220.↵
- 221.↵
- 222.↵
- 223.↵
- 224.↵
- 225.↵
- 226.↵
- 227.↵
- © 2018 by American Journal of Neuroradiology