Abstract
BACKGROUND AND PURPOSE: Cognitive improvement has been reported after carotid revascularization and attributed to treating stenosis and correcting hypoperfusion. This study investigated the effect of carotid intraplaque hemorrhage on postintervention cognition.
MATERIALS AND METHODS: In this institutional review board–approved single-center study, consecutive patients scheduled for carotid surgery were recruited for preoperative carotid MR imaging (MPRAGE) and pre- and postintervention cognitive testing using the Repeatable Battery for the Assessment of Neuropsychological Status. Pre- and postintervention scores were compared using t tests and multivariable linear regression.
RESULTS: Twenty-three participants were included, with endarterectomy performed in 20 (87%) and angioplasty/stent placement, in 3 (13%). Overall, statistically significant improvements occurred in the pre- versus postintervention mean Total Scale score (92.1 [SD, 15.5] versus 96.1 [SD, 15.8], P = .04), immediate memory index (89.4 [SD, 18.2] versus 97.7 [SD, 14.9], P < .001), and verbal index (96.1 [SD, 14.1] versus 103.0 [SD, 12.0], P = .002). Intraplaque hemorrhage (+) participants (n = 11) had no significant improvement in any category, and the attention index significantly decreased (99.4 [SD, 18.0] versus 93.5 [SD, 19.4], P = .045). Intraplaque hemorrhage (−) participants (n = 12) significantly improved in the Total Scale score (86.4 [SD, 11.8] versus 95.5 [SD, 12.4], P = .004), immediate memory index (82.3 [SD, 14.6] versus 96.2 [SD, 14.1], P = .002), delayed memory index (94.3 [SD, 14.9] versus 102.4 [SD, 8.0], P = .03), and verbal index (94.3 [SD, 13.2] versus 101.5 [SD, 107.4], P = .009). Postintervention minus preintervention scores for intraplaque hemorrhage (+) versus (−) groups showed statistically significant differences in the Total Scale score (−0.4 [SD, 6.8] versus 8.0 [SD, 8.5], P = .02), attention index (−5.9 [SD, 8.5] versus 4.3 [SD, 11.9], P = .03), and immediate memory index (4.2 [SD, 6.7] versus 12.2 [SD, 10.2], P = .04).
CONCLUSIONS: Cognitive improvement was observed after carotid intervention, and this was attributable to intraplaque hemorrhage (−) plaque. MR imaging detection of intraplaque hemorrhage status may be an important determinant of cognitive change after intervention.
ABBREVIATIONS:
- ACA
- anterior cerebral artery
- BMI
- body mass index
- IPH
- intraplaque hemorrhage
- PCA
- posterior cerebral artery
- RBANS
- Repeatable Battery for the Assessment of Neuropsychological Status
An association between dementia, carotid stenosis, and cognitive improvement following the restoration of blood flow was proposed in the early 1950s.1,2 Subsequently, carotid atherosclerosis was identified as a risk factor for dementia,3 and revascularization was linked to improved cognition.4⇓-6 Consequently, impaired hemodynamics secondary to flow-limiting stenosis was proposed as the primary mechanism for the association between carotid atherosclerosis and cognitive impairment.2 This hypothesis was supported by an association between cerebral hypoperfusion, accelerated cognitive decline, and an increased risk of dementia.7
Contrary to this, however, computational models suggested that very high-grade carotid stenosis (up to 86%) was required to reduce the cerebral perfusion pressure.8 In addition, the Framingham Study showed that lesser degrees of stenosis down to 50% were still associated with poor executive function.9 Furthermore, a study of asymptomatic severe carotid stenosis found that downstream perfusion was predominantly unaltered.10 Together, these findings suggest that an alternative etiology underpins the association between carotid atherosclerosis and cognitive decline.
Vulnerable plaque is a manifestation of advanced carotid atherosclerosis, which may play a role in cognitive decline independent of stenosis. A major determinant of vulnerable plaque is intraplaque hemorrhage (IPH), which confers an increased risk of thromboembolic stroke.11⇓-13 IPH was identified as a predictor of carotid-source stroke independent of stenosis.14 Vulnerable plaque with IPH may lead to increased cerebral microemboli, which are associated with a hastened progression of dementia.15 IPH is not only a marker of thromboembolic activity,16 but it may also influence downstream cerebral hemodynamics.17,18 Removal of vulnerable plaque has the potential to stabilize cognitive decline.19
Carotid atherosclerosis–associated cognitive decline could be multifactorial. Plaque composition may contribute in addition to the severity of the stenosis or downstream hypoperfusion. Prior to this study, the effect of IPH status on cognitive improvement after carotid surgery was unknown. This study aimed to assess the association between IPH(+) or (−) plaque and cognition following carotid revascularization. This study hypothesized that IPH status would play a role in cognitive benefit seen after carotid intervention and that removal of IPH(+) plaque would confer the greatest benefit.
MATERIALS AND METHODS
Study Design and Population
Local institutional review board (University of Utah) approval was granted for this prospective study, and informed consent was obtained. Study procedures including data acquisition and storage were compliant with the Health Insurance Portability and Accountability Act. The study protocol (ID 17SDG33460420/NCT03068442) can be viewed at https://clinicaltrials.gov.
Subjects were prospectively recruited from the neurovascular consultation and outpatient services at 2 institutions (University of Utah Medical Center and VA Salt Lake City Health Care System) between January 2017 and February 2020. Consecutive subjects with carotid disease necessitating intervention, either symptomatic with ≥1 carotid plaque with ≥50% stenosis or asymptomatic with ≥70% stenosis as per the Society for Vascular Surgery guidelines20 were included. The exclusion criteria were the following: 1) contraindication to CTA or MR imaging (unsuitable pacemaker, contrast allergy, ocular foreign body, estimated glomerular filtration rate of < 30 mL/min), 2) stage IV malignancy, and 3) known dementia (vascular dementia or any other cause, including Alzheimer disease). Additionally, subjects with known cardioembolic stroke factors (eg, mechanical valve, atrial fibrillation) were excluded to eliminate any confounding effects from these stroke etiologies. Finally, subjects with carotid occlusion were excluded because chronicity is often indeterminate and preocclusion lumen features could not be assessed. Before enrollment, all participants underwent a standard-of-care preintervention carotid CTA. After recruitment, subjects underwent a research carotid MR imaging and cognitive testing pre- and postintervention.
Clinical Characteristics
Relevant demographic and clinical characteristics were recorded following chart review. Cerebrovascular risk factors included age, sex, body mass index (BMI), smoking status, diabetes, renal insufficiency, hypertension, and hyperlipidemia. Diagnoses were assigned using standard clinical definitions. Renal insufficiency was an estimated glomerular filtration rate of < 45mL/min. Hypertension was diagnosed if the average of ≥2 diastolic blood pressure measurements on at least 2 subsequent visits was ≥90 mm Hg or the average of multiple systolic blood pressure readings on ≥2 subsequent visits was ≥140 mm Hg. Hyperlipidemia was assigned when low-density lipoprotein was >100 mg/dL. Male or female sex was self-reported. Cerebrovascular medications including antiplatelets, anticoagulants, statins, and antihypertensives were recorded.
Imaging and Postprocessing Protocols
Carotid MR Imaging.
Carotid MR imaging studies were performed with a 3T magnet (Magnetom Prisma; Siemens) using the vendor’s head and neck coil in conjunction with a dedicated 7-channel custom neck coil.21 The protocol included a TOF (axial acquisition: TR/TE = 20/3.4 ms, FOV = 240 × 240 mm2, matrix = 320 × 320 × 100, voxel = 0.77 × 0.77 × 0.77 mm3); a 3D T1-weighted MPRAGE obtained 20 mm below to 20 mm above the carotid bifurcation, 1.0-mm section thickness (coronal acquisition: TR/TE/TI = 6.39/2.37/370 ms, flip angle = 15°, FOV = 180 × 180 × 92 mm3, matrix = 320 × 320 × 120, voxel = 0.77 × 0.77 × 0.77 mm3); and a 3D T1 sampling perfection with application-optimized contrasts using different flip angle evolution (SPACE; Siemens) sequence (coronal acquisition: TR/TE = 800/22 ms, delay alternating with nutation for tailored excitation [DANTE] preparation = 150 ms, FOV = 180 × 180 × 77, matrix = 320 × 320 × 100, voxel = 0.77 × 0.77 × 0.77).
DSC Brain Imaging.
DSC was performed with an axial acquisition (TR/TE = 2070/52 ms, voxel = 2.0 × 2.0 × 2.0 mm3, 5.0-mm section thickness, 100 time points/15 sections and with 1 mmol/mL−1 of Gadubutrol [Gadavist; Bayer Schering Pharma]). DSC was preceded by an initial contrast predose to minimize errors in CBV estimates. Data were transferred to an external workstation and processed by a neuroradiologist blinded to additional imaging and clinical information using FDA-approved software (Olea Sphere, Version 3.0-SP5; Olea Medical) and automated arterial input function selection.
Imaging Analysis
Perfusion Analysis.
For each cerebral hemisphere, ROIs were outlined for the anterior cerebral artery (ACA), MCA, and posterior cerebral artery (PCA) territories using arterial territory maps for reference.22 ROI analysis was conducted at 3 different levels of the brain separated by 2 axial slices, and the average measurement of these was used. Relative CBF, CBV, and MTT were assessed for the ACA, MCA and PCA territories for the ipsilateral (side of intervention) and contralateral vascular territories. Total CBF, CBV, and MTT perfusion was the sum of all the territories (ACA, MCA, and PCA) for the ipsilateral and contralateral hemispheres. Ratios were computed for the ACA, MCA, and PCA CBF, CBV, and MTT as a ratio of ipsilateral-to-contralateral territories. Finally, the Total ratios were the total sum of all 3 hemispheric vascular territories expressed as a ratio of ipsilateral-to-contralateral hemispheres.
Carotid Plaque Features.
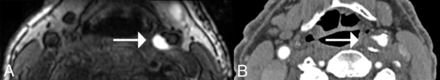
A neuroradiologist blinded to all details evaluated each MPRAGE sequence for IPH. An IPH(+) status was assigned using a ≥2-fold signal threshold over the adjacent sternocleidomastoid muscle, shown to have high interrater reliability and histologic correlation as previously described (Figure).23 Lumen measurements were the consensus of a blinded neuroradiologist and a senior neuroradiology fellow. ICA stenosis was quantified on the preintervention CTA using the NASCET criteria [(a − b)/a]×100%, where a is the ICA diameter distal to the stenosis and b is the diameter at the level of maximal stenosis.24 Maximum plaque thickness was measured on precontrast 3D T1 SPACE perpendicular to the axis of the lumen.
Carotid IPH in a 77-year-old male participant. An axial MPRAGE (A) shows IPH(+) eccentric plaque at the left carotid bulb (arrow). The avid hyperintense signal on this heavily T1-weighted sequence is due to methemoglobin in the blood products. Axial CTA (B) shows the corresponding CTA appearance of this plaque. The predominantly noncalcified plaque (arrow) narrowed the vessel lumen by 62%.
Ischemic Stroke Evaluation
Ischemic stroke was defined according to the American Heart Association definition of CNS infarction as brain or retinal cell death attributable to ischemia based on imaging evidence of cerebral or retinal ischemia in the carotid distribution or clinical symptoms persisting for ≥24 hours, with other etiologies excluded.25 Preceding intervention, ischemic stroke status was determined by neurologic examination supplemented with brain DWI concurrently performed during each research carotid MR imaging. DWI was reviewed by a blinded neuroradiologist for recent infarcts. DWI trace (hyperintense) and ADC (hypointense) were the imaging determinants of recent infarction.26,27
WM Disease Rating
WM disease was assessed on T2-weighted FLAIR sequences from MR imaging brain studies using the Age-Related White Matter Changes rating scale.28 Ratings were performed independently by a neuroradiologist blinded to cognitive and plaque results. WM changes were defined as hyperintense lesions of ≥5 mm on T2 or FLAIR, and lacunes were well-defined areas of >2 mm.28 The basal ganglia, and frontal, parieto-occipital, and temporal WM were rated on a 4-point scale and presented as a total score.28
Repeatable Battery for the Assessment of Neuropsychological Status Cognitive Testing
The repeatable Battery for the Assessment of Neuropsychological Status (RBANS) is a brief cognitive battery of 12 subtests, yielding 5 index scores: immediate memory index (list learning, story memory), visuospatial/constructional index (figure copy, line orientation), language index (picture naming, semantic fluency), attention index (digit span, coding), delayed memory index (list recall, list recognition, story recall, figure recall), and a Total Scale score (all subtests).29 Subtests were administered and scored according to the RBANS test manual, except for figure copy and figure recall subtests, which were scored with a modified set of scoring criteria.30, 31
Data are presented as raw scores for the subtests, and the index and Total Scale scores are age-corrected standard scores (mean = 100 [SD, 15]) based on normative data in the test manual. Two additional indexes, visual and verbal indexes, were generated.32 Subtest scores were converted to age- and education-corrected scaled scores (mean = 10 [SD , 3]) using Table 2 from Duff et al.32 These age- and education-corrected scaled scores were used to generate the verbal index (list learning, story memory, list recall, list recognition, story recall) and visual index (figure copy, line orientation, coding, and figure recall).30
RBANS has been previously described for subjects undergoing carotid endarterectomy33,34 and was administered preintervention and at least 1-month postintervention. The effects of hospitalization, anesthesia, and surgery should have been resolved by approximately 30 days postoperatively.35 A research assistant administered alternate RBANS forms at visits to reduce practice effects. Across all scores (eg, raw, scaled, standard scores), higher scores indicate better cognitive performance. Baseline cognitive impairment was defined as a RBANS-1 score of ≤80 (>1.3 SDs below the mean). RBANS interpretation was overseen by a neuropsychologist blinded to all patient information.
Reliable Change Assessment.
Within neuropsychology, reliable change methods attempt to determine clinically meaningful cognitive change. These neuropsychology methods try to distinguish whether changes across 2 RBANS test sessions are the result of normal variations in the patient’s performance and testing methods or are indeed consequential cognitive changes.36 Factors that can affect cognitive testing results such as differential practice effects, systematic biases, and measurement error are taken into account.36 Using regression-based prediction algorithms, predicted scores can be made for each patient’s RBANS indexes and subtest scores.36 Predicted postrevascularization scores, RBANS-2PD, were generated with the prediction algorithms (derived from a sample of 129 cognitively intact older adults, mean age = 75.6 [SD, 7.5] years).36 These scores (RBANS-2PD) represent the expected RBANS scores at follow-up for each patient. Predicted RBANS-2PD scores were subsequently compared with the actual observed scores of the study, RBANS-2OB, by subtracting the predicted from the observed scores (RBANS-2OB minus RBANS-2PD).
Statistical Analysis
Continuous variables were expressed as mean (SD), and categoric variables, as frequencies. Two-sided t tests and χ2 tests or Fisher exact tests were used to compare IPH groups. Univariable linear regression tested the association between the outcome variable (RBANS scores) and each covariate. Potential confounding variables were those covariates with P < .20 in univariable regression, and these were subsequently assessed using multivariable linear regression. For each multivariable linear regression model, the outcome of interest was ΔRBANS (RBANS-2 minus RBANS-1), and IPH was the primary predictor variable. Each covariate was eliminated using backwards elimination until all remaining variables met a P < .10 threshold. Consideration was given to the number of covariates to avoid overfitting the models. Analyses were performed using STATA software (Version 17.1; StataCorp).
RESULTS
Participant, Plaque, and Perfusion Characteristics
Whole Group.
Twenty-three participants (mean age, 66.2 [SD, 8.0] years; 21 men [91.3%]) were recruited and included in the analyses. The mean percentage diameter stenosis on the side of the intervention was 68.0% (SD, 18.9%). Most participants, 16 (69.6%), were asymptomatic preceding the intervention. Eleven (47.8%) were IPH(+). The right ICA was the predominant carotid artery intervened on for 14 (60.9%) participants. All subjects were initially planned for endarterectomy, and the final decision on the method of intervention was endarterectomy in 20 (87%) and angioplasty/stent placement in 3 (13%). Procedures were performed by senior vascular surgeons with a mean of 20.3 (SD, 33.6) years of experience and lasted, on average, a mean of 133.1 (SD, 3.6) minutes. RBANS-1 test scores showed that 6 (26.1%) participants were cognitively impaired at baseline. Clinical characteristics are summarized in Table 1, and carotid plaque, perfusion, and cognition are shown in Table 2.
Clinical characteristicsa
Plaque, perfusion, and RBANS test characteristicsa
IPH(+) and IPH(–) Groups.
Two parameters were significantly different between the IPH (+) and (−) groups: age and MTT Total. Participants with IPH(+) were older at a mean age of 71.2 (SD, 7.6) years compared with 61.5 (SD, 5.2) years (P = .002). The MTT Total was significantly more delayed in the presence of IPH (mean = 9.0 [SD, 2.0] versus 7.6 [SD, 1.2], P = .049). WM disease ratings, ischemic stroke evaluation, and baseline cognition were not statistically significant between the 2 groups. Additionally, all remaining clinical, plaque, perfusion, procedural, and cognitive parameters (Tables 1 and 2) were not significantly different between the IPH groups at baseline.
Baseline and Postintervention Cognitive Assessment
Whole-Group Analysis.
RBANS domains were compared before (RBANS-1) and after (RBANS-2) the carotid intervention (Table 3). RBANS-1 was performed preintervention at a mean of 28.5 (SD, 23.5) days (range, 1–90 days) and postintervention RBANS-2 at 266.6 (SD, 258.1) days (range, 53–838 days). Whole-group analysis revealed a statistically significant improvement from baseline in 3 domains: mean Total Scale score (RBANS-1, 92.1 [SD, 15.5] versus RBANS-2, 96.1 [SD, 15.8], P = .04); immediate memory index (RBANS-1, 89.4 [SD, 18.2] versus RBANS-2, 97.7 [SD, 14.9], P < .001), and verbal index (RBANS-1, 96.1 (SD, 14.1) versus RBANS-2, 103.0 [SD, 12.0], P = .002). The remaining indexes were not significantly changed after the intervention.
Mean baseline and postintervention RBANS scores
IPH Status and Postintervention Cognition
Analysis by IPH Status.
RBANS-1 and RBANS-2 mean scores were compared between IPH(+) (n = 11) and IPH(−) (n = 12) groups (Online Supplemental Data). The range during which the RBANS-2 testing occurred was not significantly different between the IPH(+) and (−) groups. The IPH(+) group had no significant improvement in postintervention scores. One index, the attention index, significantly decreased from baseline (RBANS-1, 99.4 [SD, 18.0] versus RBANS-2, 93.5 [SD, 19.4], P = .045). Postintervention, the IPH(−) group significantly improved in 4 scores: Total Scale score (RBANS-1, 86.4 [SD, 11.8] versus RBANS-2, 95.5 [SD, 12.4], P = .004), immediate memory index (RBANS-1, 82.3 [SD, 14.6] versus RBANS-2, 96.2 [SD, 14.1], P = .002), delayed memory index (RBANS-1, 94.3 [SD, 14.9] versus RBANS-2, 102.4 [SD, 8.0], P = .03), and verbal index (RBANS-1, 94.3 [SD, 13.2] versus RBANS-2, 101.5 [SD, 107.4], P = .009). Subtracted mean scores (RBANS-2 minus RBANS-1) for each domain were designated ΔRBANS. IPH(+) and (−) groups had statistically significant differences in the ΔRBANS Total Scale score: IPH(+), −0.4 (SD, 6.8) versus IPH(−), 8.0 (SD, 8.5), P = .02; attention index, IPH(+), −5.9 (SD, 8.5) versus IPH(−), 4.3 (SD, 11.9), P = .03; and immediate memory index IPH(+), 4.2 (SD, 6.7) versus IPH(−), 12.2 (SD, 10.2), P = .04.
Multivariable Analysis of Postintervention Cognitive Outcomes
Multivariable regression models were fitted to the 3 cognitive outcomes with significantly different ΔRBANS scores between subjects with IPH(+) and (−): ΔRBANS Total Scale score, attention index, and immediate memory index. The ΔRBANS Total Scale score final model consisted of IPH(+) plaque (β = −6.17; 95% CI, −12.49–0.15; P = .06) and hyperlipidemia (β = −9.05; 95% CI, −16.70 to −1.40; P = .02). The ΔRBANS attention index final model included only IPH(+) plaque (β = −10.24; 95% CI, −19.29 to −1.20; P = .03). The final model for ΔRBANS immediate memory index included IPH(+) plaque (β = −8.17; 95% CI, −15.10 to −1.24; P = .02), BMI (β = 0.49; 95% CI, 0.02–0.97; P = .04), and the MTT Total ratio (β = −48.51; 95% CI, −100.70–3.69; P = .07). All univariable and multivariable regression analyses are shown in Online Supplemental Data.
Reliable Change Assessment
Predicted RBANS-2 scores (RBANS-2PD) were generated (Online Supplemental Data). Observed RBANS-2 scores (RBANS-2OB) were subtracted from the predicted (RBANS-2PD) for the whole group, GroupOB-PD, and according to IPH status, IPH(+)OB-PD and IPH(−)OB-PD. For the group, the attention index groupOB-PD (mean, −6.3 [SD, 11.2], P = .01), immediate memory index groupOB-PD (mean, −5.0 [SD, 8.1], P = .007), and language index groupOB-PD (mean, −3.7 [SD, 6.7], P = .02) significantly deviated from predicted scores. IPH(+) status showed a significantly lower-than-expected attention index IPH(+)OB-PD (mean, −8.9 [SD, 10.5], P = .02) and immediate memory index IPH(+)OB-PD (mean, −5.3 [SD, 6.8], P = .026). The IPH(−) group’s language index significantly deviated from predicted, IPH(−)OB-PD (mean, −5.2 [SD, 6.1], P = .01).
DISCUSSION
This study examined the changes in cognition after carotid plaque removal and the impact of IPH status on postintervention scores. The effects of stenosis, perfusion, and additional potential confounders were also evaluated. Whole-group analysis showed improvement in three domains: the overall Total Scale score, immediate memory index, and verbal index following intervention. Subgroup evaluation indicated that preintervention IPH status impacted postintervention cognition. An IPH(−) status conferred the most cognitive benefit with a significantly improved Total Scale score, immediate memory index, delayed memory index, and verbal index. For IPH(+) participants, there was no statistically significant improvement in any of the scores, and the attention index declined.
The whole group’s improved cognitive performance is in keeping with prior studies that also showed an association between carotid revascularization and improved cognition.2,33,34,37 Takaiwa et al33,34 demonstrated an improved RBANS Total Scale score and immediate memory index 3 months postcarotid endarterectomy, which was sustained after 1 year. Takaiwa et al additionally reported an improved attention index.33,34 In the present study, attention index performance differed according to IPH status. The IPH(−) group’s attention index increased, though not significantly. Conversely, the IPH(+) group’s score significantly decreased. This finding suggests that IPH could differentially affect some cognitive domains more than others.
The present study evaluated 2 additional indexes that may help to lateralize pathology. The verbal and visual indexes should be most representative of the left and right cerebral hemispheric function, respectively.32,38 Revascularization was most beneficial for the verbal index, which increased for the whole group, again attributable to those with IPH(−) plaque; however, the visual index remained unchanged. Improved verbal scores would be expected more with a left-sided intervention.32,38,39 In the current study, however, this finding was not explained by the side of revascularization. One possibility is that carotid disease may affect functional brain connectivity beyond the ipsilateral vascular territory.40
Prior studies that examined postintervention cognitive effects had not considered plaque composition.2,33,34,37 At the outset, this study hypothesized that intervention on IPH(+) plaque should have the greatest cognitive benefit, given its underlying thromboembolic activity,16 risk of stroke, and TIA.11⇓-13 Instead, the converse occurred, and intervention on IPH(−) plaque ameliorated 4 cognitive outcomes (Total Scale score, attention index, immediate index, and verbal index). One explanation for the less-than-expected IPH(+) group’s performance is microembolization during the intervention.41 An association between vulnerable plaque and postintervention ischemic events has been previously shown.42⇓⇓-45 During carotid endarterectomy, IPH increased the embolization risk, specifically during the dissection phase.43 After stent placement, a higher risk of ipsilateral ischemic events was found and correlated with IPH volume.44,45 In the current study, no participant had a clinically evident postprocedural ischemic stroke; however, periprocedural monitoring for silent emboli or postprocedural MR imaging for covert infarction was not performed. Cognitive improvement with IPH(−) plaque could be attributable to plaque composition. Ulceration and intraluminal thrombus, both features of plaque instability, were not statistically different between the IPH groups. However, other plaque constituents or morphologic features including calcification or a lipid-rich necrotic core may be implicated in the amelioration of some IPH(−) cognitive domains.
Confounding was addressed during statistical analysis. Three confounders were identified; 2 (hyperlipidemia and MTT Total ratio) adversely affected cognition, while 1 (BMI) had a positive association. Of the cardiovascular risk factors, only hyperlipidemia was negatively associated with the Total Scale score. High cholesterol is a known risk factor for cognitive impairment.46,47 Secondly, the MTT Total ratio negatively impacted the immediate memory index and was statistically longer for the IPH(+) participants. MTT measures the average time of red blood cells in the capillary circulation and can indicate impaired perfusion. In addition to steno-occlusive disease, perfusion could be influenced by plaque components including IPH volume.17 BMI was positively associated with the attention index. At an older age, a high BMI could have a protective effect against the progression of dementia.48⇓-50 A survivorship bias effect is one plausible explanation for this obesity-dementia paradox.51
In a further attempt to gauge the benefit of IPH removal, the reliable change methodology was used. Baseline scores were used to estimate expected follow-up cognition on the basis of predictions derived for healthy age- and education-matched community dwellers.36 This was performed to indicate whether a change in a score was statistically different from what was expected at follow-up. At baseline, participants’ scores were predominantly in the RBANS average range, with a small number cognitively impaired; however, the number with cognitive impairment was not statistically different between the groups. After the intervention, the IPH(+) group had a lower-than-predicted attention index and immediate memory index. For IPH(−) participants, the language index was lower than predicted. Findings suggest that despite intervention, some cognitive abilities were unrecoverable for both groups, and the IPH(+) group was most affected. This outcome could be related to the long-standing impact on neuroplasticity from recurrent microembolization.
This study has some limitations. The sample size was small, though this limitation was comparable with that in similar studies of postintervention carotid disease and cognition.33,34 Despite no enrollment restrictions on demographics, the group were all white and predominantly male, which is a known population at risk of carotid disease.52 Accordingly, ethnicity and sex influences were unattainable. The effect of periprocedural microembolization was not examined, and routine postprocedural MR imaging screening was not conducted. Emboli monitoring or postintervention MR imaging could further address the hypotheses of an embolic-driven mechanism of cognitive impairment. Baseline cognition was not compared with a randomized control group; however, the predicted score calculations enabled comparison with a group of community-dwelling older adults.36
CONCLUSIONS
Despite these limitations, this study was a critical initial step toward elucidating the effect of carotid IPH on cognition after revascularization. While the management of carotid bifurcation stenosis has been extensively investigated with recommendations for management,20 guidelines regarding the role of cognition in decision-making or stratifying patients have yet to be established. Additionally, an extended follow-up period would evaluate the long-term stability of postintervention cognition changes. Future studies are warranted to further understand the association between plaque composition and cognition.
Footnotes
This work was supported by the American Heart Association Scientist Development Grant 17SDG33460420 (J.S. McNally) and the National Institutes of Health R01 HL127582 (J.S. McNally).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received May 11, 2022.
- Accepted after revision October 1, 2022.
- © 2022 by American Journal of Neuroradiology