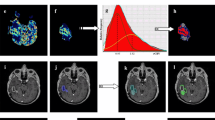
Abstract
Introduction
The interest in measuring brain perfusion with intravoxel incoherent motion (IVIM) MRI has significantly increased in the last 3 years. Our aim was to evaluate the prognostic value for survival of intravoxel incoherent motion perfusion fraction in patients with gliomas, and compare it to dynamic susceptibility contrast relative cerebral blood volume and apparent diffusion coefficient.
Methods
Images were acquired in 27 patients with brain gliomas (16 high grades, 11 low grades), before any relevant treatment. Region of maximal perfusion fraction, maximal relative cerebral blood volume, and minimal apparent diffusion coefficient were obtained. The accuracy of all three methods for 2‑year survival prognosis was compared using the area under the receiver operating characteristic curve and Kaplan–Meier survival curves.
Results
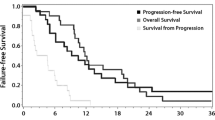
Death or survival for at least 2 years after imaging could be documented in 22/27 patients. The cutoff values of 0.112 for the perfusion fraction, of 3.01 for the relative cerebral blood volume, and 1033 × 10−6 mm2/s for apparent diffusion coefficient led to an identical sensitivity of 0.889, and a specificity of 0.833, 0.517, and 0.750, respectively for 2 year survival prognosis. The corresponding areas under the receiver operating characteristic curves were 0.84, 076, and 0.86, respectively. All three methods had a significant log rank test considering overall survival (p = 0.001, p = 0.028, and p = 0.002).
Conclusion
In this relatively small cohort, maximal IVIM perfusion fraction, similarly to maximal relative cerebral blood volume and minimal apparent diffusion coefficient, was prognostic for survival in patients with gliomas. Maximal IVIM perfusion fraction and minimal apparent diffusion coefficient performed similarly in predicting survival, and both slightly outperformed maximal relative cerebral blood volume.




Similar content being viewed by others
Abbreviations
- IVIM:
-
intravoxel incoherent motion
- rCBV:
-
relative cerebral blood volume
- D:
-
diffusion coefficient
- f:
-
perfusion fraction
References
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(Suppl 4):iv1–63.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96.
Aronen HJ, Gazit IE, Louis DN, Buchbinder BR, Pardo FS,Weisskoff RM, Harsh GR, Cosgrove GR, Halpern EF, Hochberg FH, et al. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology. 1994;191:41–51.
Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, Knopp EA, Zagzag D. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003;24:1989–98.
Law M, Young RJ, Babb JS, Peccerelli N, Chheang S, Gruber ML, Miller DC, Golfinos JG, Zagzag D, Johnson G. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2008;247:490–8.
Hilario A, Sepulveda JM, Perez-Nuñez A, Salvador E, Millan JM, Hernandez-Lain A, Rodriguez-Gonzalez V, Lagares A, Ramos A. A prognostic model based on preoperative MRI predicts overall survival in patients with diffuse gliomas. AJNR Am J Neuroradiol. 2014;35:1096–1102.
Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497–505.
Federau C, Hagmann P, Maeder P, Müller M, Meuli R, Stuber M, O’Brien K. Dependence of brain intravoxel incoherent motion perfusion parameters on the cardiac cycle. PLoS ONE. 2013;8:e72856.
Federau C, Maeder P, O’Brien K, Browaeys P, Meuli R, Hagmann P. Quantitative measurement of brain perfusion with intravoxel incoherent motion MR imaging. Radiology. 2012;265:874–81.
Federau C, O’Brien K, Meuli R, Hagmann P, Maeder P. Measuring brain perfusion with intravoxel incoherent motion (IVIM): initial clinical experience. J Magn Reson Imaging. 2014;39:624–32.
Federau C, Meuli R, O’Brien K, Maeder P, Hagmann P. Perfusion measurement in brain gliomas with intravoxel incoherent motion MRI. AJNR Am J Neuroradiol. 2014;35:256–62.
Bisdas S, Koh TS, Roder C, Braun C, Schittenhelm J, Ernemann U, Klose U. Intravoxel incoherent motion diffusion-weighted MR imaging of gliomas: feasibility of the method and initial results. Neuroradiology. 2013;55:1189–96.
Suh CH, Kim HS, Lee SS, Kim N, Yoon HM, Choi CG, Kim SJ. Atypical imaging features of primary central nervous system lymphoma that mimics glioblastoma: utility of intravoxel incoherent motion MR imaging. Radiology. 2014;272:504–13.
Kim HS, Suh CH, Kim N, Choi CG, Kim SJ. Histogram analysis of intravoxel incoherent motion for differentiating recurrent tumor from treatment effect in patients with glioblastoma: initial clinical experience. AJNR Am J Neuroradiol. 2014;35:490–7.
Kim DY, Kim HS, Goh MJ, Choi CG, Kim SJ. Utility of intravoxel incoherent motion MR imaging for distinguishing recurrent metastatic tumor from treatment effect following gamma knife radiosurgery: initial experience. AJNR Am J Neuroradiol. 2014;35:2082–90.
Federau C, O’Brien K, Birbaumer A, Meuli R, Hagmann P, Maeder P. Functional mapping of the human visual cortex with intravoxel incoherent motion MRI. PLoS ONE. 2015;10:e0117706.
Stejskal EO, Tanner JE. Spin Diffusion Measurements: Spin Echoes in the Presence of a Time-Dependent Field Gradient. J Chem Phys. 1965;42:288–92.
Levenberg K. A Method for the Solution of Certain Non-Linear Problems in Least Squares. Q Appl Math. 1944;2:164–8.
Marquardt D. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. SIAM J Appl Math. 1963;11:431–41.
Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol. 2006;27:859–67.
Daniel P. Barboriak Laboratory, Duke University School of Medicine, Durham, NC, USA. https://sites.duke.edu/dblab/dscoman/. Accessed March 24 2015.
Cui Y, Ma L, Chen X, Zhang Z, Jiang H, Lin S. Lower apparent diffusion coefficients indicate distinct prognosis in low-grade and high-grade glioma. J Neurooncol. 2014;119:377–85.
Higano S, Yun X, Kumabe T, Watanabe M, Mugikura S, Umetsu A, Sato A, Yamada T, Takahashi S. Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology. 2006;241:839–46.
Murakami R, Sugahara T, Nakamura H, Hirai T, Kitajima M, Hayashida Y, Baba Y, Oya N, Kuratsu J, Yamashita Y. Malignant supratentorial astrocytoma treated with postoperative radiation therapy: prognostic value of pretreatment quantitative diffusion-weighted MR imaging. Radiology. 2007;243:493–9.
Yamasaki F, Sugiyama K, Ohtaki M, Takeshima Y, Abe N, Akiyama Y, Takaba J, Amatya VJ, Saito T, Kajiwara Y, Hanaya R, Kurisu K. Glioblastoma treated with postoperative radio-chemotherapy: prognostic value of apparent diffusion coefficient at MR imaging. Eur J Radiol. 2010;73:532–7.
Zulfiqar M, Yousem DM, Lai H. ADC values and prognosis of malignant astrocytomas: does lower ADC predict a worse prognosis independent of grade of tumor? – a meta-analysis. AJR Am J Roentgenol. 2013;200:624–9.
Hilario A, Ramos A, Perez-Nuñez A, Salvador E, Millan JM, Lagares A, Sepulveda JM, Gonzalez-Leon P, Hernandez-Lain A, Ricoy JR. The added value of apparent diffusion coefficient to cerebral blood volume in the preoperative grading of diffuse gliomas. AJNR Am J Neuroradiol. 2012;33:701–7.
Zacharaki EI, Morita N, Bhatt P, O’Rourke DM, Melhem ER, Davatzikos C. Survival analysis of patients with high-grade gliomas based on data mining of imaging variables. AJNR Am J Neuroradiol. 2012;33:1065–71.
Mills SJ, Patankar TA, Haroon HA, Baleriaux D, Swindell R, Jackson A. Do cerebral blood volume and contrast transfer coefficient predict prognosis in human glioma? AJNR Am J Neuroradiol. 2006;27:853–8.
Thomsen HS. Nephrogenic systemic fibrosis: history and epidemiology. Radiol Clin North Am. 2009;47:827–31, vi.
Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC. Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol. 2014;49:685–90.
McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, Eckel LJ. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology. 2015;275:772–82.
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270:834–41.
Pekar J, Moonen CT, van Zijl PC. On the precision of diffusion/perfusion imaging by gradient sensitization. Magn Reson Med. 1992;23:122–9.
Federau C, O’Brien K. Increased brain perfusion contrast with T(2)-prepared intravoxel incoherent motion (T2prep IVIM) MRI. NMR Biomed. 2015;28:9–16.
Le Bihan D, Turner R. The capillary network: a link between IVIM and classical perfusion. Magn Reson Med. 1992;27:171–8.
Wetscherek A, Stieltjes B, Laun FB. Flow-compensated intravoxel incoherent motion diffusion imaging. Magn Reson Med. 2015;74:410–9.
Acknowledgements
C. Federau is supported by the Swiss National Science Foundation. This work was supported by the Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL, and the Leenaards and Jeantet Foundations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C. Federau, M. Cerny, M. Roux, P.J. Mosimann, P. Maeder, Reto Meuli, and M. Wintermark state that there are no conflicts of interest.
All studies on humans described in the present manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form) Informed consent was waived
Additional information
Authors’ contributions C. Federau: study design, data analysis, statistical analysis, manuscript writing; M. Cerny: data collection; M. Roux: data collection; P. Mosimann: manuscript editing; P. Maeder: manuscript editing; R. Meuli: manuscript editing; M. Wintermark: manuscript editing.
Rights and permissions
About this article
Cite this article
Federau, C., Cerny, M., Roux, M. et al. IVIM perfusion fraction is prognostic for survival in brain glioma. Clin Neuroradiol 27, 485–492 (2017). https://doi.org/10.1007/s00062-016-0510-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-016-0510-7