Abstract
Objectives
To compare contrast effects of gadobutrol with gadoterate meglumine for brain MRI in multiple sclerosis (MS) in a multicentre, randomized, prospective, intraindividual study at 3 T.
Methods
Institutional review board approval was obtained. Patients with known or suspected active MS lesions were included. Two identical MRIs were performed using randomized contrast agent order. Four post-contrast T1 sequences were acquired (start time points 0, 3, 6 and 9 min). If no enhancing lesion was present in first MRI, second MRI was cancelled. Quantitative (number and signal intensity of enhancing lesions) and qualitative parameters (time points of first and all lesions enhancing; subjective preference regarding contrast enhancement and lesion delineation; global preference) were evaluated blinded.
Results
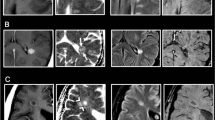
Seventy-four patients (male, 26; mean age, 35 years) were enrolled in three centres. In 45 patients enhancing lesions were found. Number of enhancing lesions increased over time for both contrast agents without significant difference (median 2 for both). Lesions signal intensity was significantly higher for gadobutrol (p < 0.05 at time points 3, 6 and 9 min). Subjective preference rating showed non-significant tendency in favour of gadobutrol.
Conclusion
Both gadobutrol and gadoterate meglumine can be used for imaging of acute inflammatory MS lesions. However, gadobutrol generates higher lesion SI.
Key Points
• Contrast-enhanced MRI plays a key role in the management of multiple sclerosis.
• Different gadolinium-based contrast agents are available.
• Number of visibly enhancing lesions increases over time after contrast injection.
• Gadobutrol and gadoterate meglumine do not differ in number of visible lesions.
• Gadobutrol generates higher signal intensity than gadoterate meglumine.





Similar content being viewed by others
Abbreviations
- BBBD:
-
blood–brain barrier disruption
- DCE-MRI:
-
dynamic contrast-enhanced MRI
- GBCA:
-
gadolinium-based contrast agent
- MRI:
-
magnetic resonance imaging
- MS:
-
multiple sclerosis
- SE:
-
spin echo
- SI:
-
signal intensity
- TE:
-
echo time
- TR:
-
repetition time
References
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302
Montalban X, Tintoré M, Swanton J et al (2010) MRI criteria for MS in patients with clinically isolated syndromes. Neurology 74:427–434
Filippi M, Preziosa P, Rocca MA (2014) Magnetic resonance outcome measures in multiple sclerosis trials: time to rethink? Curr Opin Neurol 27:290–299
Sormani MP, Bonzano L, Roccatagliata L, Cutter GR, Mancardi GL, Bruzzi P (2009) Magnetic resonance imaging as a potential surrogate for relapses in multiple sclerosis: a meta-analytic approach. Ann Neurol 65:268–275
Sormani MP, Bruzzi P (2013) MRI lesions as a surrogate for relapses in multiple sclerosis: a meta-analysis of randomised trials. Lancet Neurol 12:669–676
Koenig M, Schulte-Altedorneburg G, Piontek M et al (2013) Intra-individual, randomised comparison of the MRI contrast agents gadobutrol versus gadoteridol in patients with primary and secondary brain tumours, evaluated in a blinded read. Eur Radiol 23:3287–3295
Anzalone N, Scarabino T, Venturi C et al (2013) Cerebral neoplastic enhancing lesions: multicenter, randomized, crossover intraindividual comparison between gadobutrol (1.0M) and gadoterate meglumine (0.5M) at 0.1 mmol Gd/kg body weight in a clinical setting. Eur J Radiol 82:139–145
Anzalone N, Gerevini S, Scotti R, Vezzulli P, Picozzi P (2009) Detection of cerebral metastases on magnetic resonance imaging: intraindividual comparison of gadobutrol with gadopentetate dimeglumine. Acta Radiol 50:933–940
European Society of Urogenital Radiology (2014) ESUR guidelines on contrast media. European Society of Urogenital Radiology, Vienna. Available via http://www.esur.org/guidelines/. Accessed 15 Mar 2015
Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ (2005) Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Investig Radiol 40:715–724
Wansapura JP, Holland SK, Dunn RS, Ball WS Jr (1999) NMR relaxation times in the human brain at 3.0 tesla. J Magn Reson Imaging 9:531–538
Filippi M, Yousry T, Rocca MA, Fesl G, Voltz R, Comi G (1997) Sensitivity of delayed gadolinium-enhanced MRI in multiple sclerosis. Acta Neurol Scand 95:331–334
Simon JH, Li D, Traboulsee A et al (2006) Standardized MR imaging protocol for multiple sclerosis: consortium of MS Centers consensus guidelines. AJNR Am J Neuroradiol 27:455–461
Lövblad KO, Anzalone N, Dörfler A et al (2010) MR imaging in multiple sclerosis: review and recommendations for current practice. AJNR Am J Neuroradiol 31:983–989
Heye AK, Culling RD, Valdés Hernández MD, Thrippleton MJ, Wardlaw JM (2014) Assessment of blood–brain barrier disruption using dynamic contrast-enhanced MRI. A systematic review. Neuroimage Clin 6:262–274
Jelescu IO, Leppert IR, Narayanan S, Araújo D, Arnold DL, Pike GB (2011) Dual-temporal resolution dynamic contrast-enhanced MRI protocol for blood–brain barrier permeability measurement in enhancing multiple sclerosis lesions. J Magn Reson Imaging 33:1291–1300
Leppert IR, Narayanan S, Araújo D et al (2014) Interpreting therapeutic effect in multiple sclerosis via MRI contrast enhancing lesions: now you see them, now you don't. J Neurol 261:809–816
Stankiewicz JM, Glanz BI, Healy BC et al (2011) Brain MRI lesion load at 1.5T and 3T versus clinical status in multiple sclerosis. J Neuroimaging 21:e50–e56
Uysal E, Erturk SM, Yildirim H, Seleker F, Basak M (2007) Sensitivity of immediate and delayed gadolinium-enhanced MRI after injection of 0.5 M and 1.0 M gadolinium chelates for detecting multiple sclerosis lesions. AJR Am J Roentgenol 188:697–702
Filippi M, Rovaris M, Capra R et al (1998) A multi-centre longitudinal study comparing the sensitivity of monthly MRI after standard and triple dose gadolinium-DTPA for monitoring disease activity in multiple sclerosis. Implications for phase II clinical trials. Brain 121:2011–2020
Le Duc G, Corde S, Charvet AM et al (2004) In vivo measurement of gadolinium concentration in a rat glioma model by monochromatic quantitative computed tomography: comparison between gadopentetate dimeglumine and gadobutrol. Investig Radiol 39:385–393
Silver NC, Good CD, Barker GJ et al (1997) Sensitivity of contrast enhanced MRI in multiple sclerosis. Effects of gadolinium dose, magnetization transfer contrast and delayed imaging. Brain 120:1149–1161
Engelhorn T, Schwarz MA, Eyupoglu IY, Kloska SP, Struffert T, Doerfler A (2010) Dynamic contrast enhancement of experimental glioma an intra-individual comparative study to assess the optimal time delay. Acad Radiol 17:188–193
Bagheri MH, Meshksar A, Nabavizadeh SA, Borhani-Haghighi A, Ashjazadeh N, Nikseresht AR (2008) Diagnostic value of contrast-enhanced fluid-attenuated inversion-recovery and delayed contrast-enhanced brain MRI in multiple sclerosis. Acad Radiol 15:15–23
Hashemi H, Behzadi S, Ghanaati H et al (2014) Evaluation of plaque detection and optimum time of enhancement in acute attack multiple sclerosis after contrast injection. Acta Radiol 55:218–224
Filippi M, Capra R, Campi A et al (1996) Triple dose of gadolinium-DTPA and delayed MRI in patients with benign multiple sclerosis. J Neurol Neurosurg Psychiatry 60:526–530
Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H (2000) Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 47:707–717
Rosenberg GA (2012) Neurological diseases in relation to the blood–brain barrier. J Cereb Blood Flow Metab 32:1139–1151
Soon D, Tozer D, Altmann D, Tofts P, Miller D (2007) Quantification of subtle blood–brain barrier disruption in non-enhancing lesions in multiple sclerosis: a study of disease and lesion subtypes. Mult Scler 13:884–894
Kermode AG, Thompson AJ, Tofts P et al (1990) Breakdown of the blood–brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis. Pathogenetic and clinical implications. Brain 113:1477–1489
Tofts PS, Kermode AG (1991) Measurement of the blood–brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med 17:357–367
Ingrisch M, Sourbron S, Morhard D et al (2012) Quantification of perfusion and permeability in multiple sclerosis: dynamic contrast-enhanced MRI in 3D at 3T. Investig Radiol 47:252–258
Acknowledgments
The scientific guarantor of this publication is Prof. Dr. A. Doerfler. The authors of this manuscript declare relationships with the following companies: Carsten Schwenke works as a freelance statistician for Bayer on an honorary basis. Norbert Hosten is a stockholder of Siemens and is an institutional grant recipient of Siemens and Bayer. This study has received funding by Bayer. One of the authors has significant statistical expertise. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. No study subjects or cohorts have been previously reported. Methodology: prospective, randomised controlled trial, multicentre study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saake, M., Langner, S., Schwenke, C. et al. MRI in multiple sclerosis: an intra-individual, randomized and multicentric comparison of gadobutrol with gadoterate meglumine at 3 T. Eur Radiol 26, 820–828 (2016). https://doi.org/10.1007/s00330-015-3889-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3889-7