Abstract
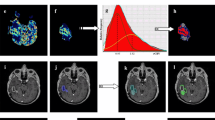
A subset of patients with high-grade glioma and brain metastases who are treated with bevacizumab develop regions of marked and persistent restricted diffusion that do not reflect recurrent tumor. Here, we quantify the degree of restricted diffusion and the relative cerebral blood volume (rCBV) within these regions of bevacizumab-related imaging abnormality (BRIA) in order to facilitate differentiation of these lesions from recurrent tumor. Six patients with high-grade glioma and two patients with brain metastases who developed regions of restricted diffusion after initiation of bevacizumab were included. Six pre-treatment GBM controls were also included. Restriction spectrum imaging (RSI) was used to create diffusion maps which were co-registered with rCBV maps. Within regions of restricted diffusion, mean RSI values and mean rCBV values were calculated for patients with BRIA and for the GBM controls. These values were also calculated for normal-appearing white matter (NAWM). RSI values in regions of restricted diffusion were higher for both BRIA and tumor when compared to NAWM; furthermore RSI values in BRIA were slightly higher than in tumor. Conversely, rCBV values were very low in BRIA—lower than both tumor and NAWM. However, there was only a trend for rCBV values to be higher in tumor than in NAWM. When evaluating areas of restricted diffusion in patients with high-grade glioma or brain metastases treated with bevacizumab, RSI is better able to detect the presence of pathology whereas rCBV is better able to differentiate BRIA from tumor. Thus, combining these tools may help to differentiate necrotic tissue related to bevacizumab treatment from recurrent tumor.




Similar content being viewed by others
Abbreviations
- BRIA:
-
Bevacizumab-related imaging abnormality
- GBM:
-
Glioblastoma multiforme
- NAWM:
-
Normal-appearing white matter
- NSCLC:
-
Non-small cell lung cancer
- rCBV:
-
Relative cerebral blood volume
- RSI:
-
Restriction spectrum imaging
- VEGF:
-
Vascular endothelial growth factor
References
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Soffietti R, Trevisan E, Ruda R (2012) Targeted therapy in brain metastasis. Curr Opin Oncol 24:679–686
Schettino C, Bareschino MA, Rossi A et al (2012) Targeting angiogenesis for treatment of NSCLC brain metastases. Curr Cancer Drug Targets 12:289–299
Hygino da Cruz LC Jr, Rodriguez I, Domingues RC et al (2011) Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. Am J Neuroradiol (AJNR) 32:1978–1985
Jeyaretna DS, Curry WT Jr, Batchelor TT et al (2011) Exacerbation of cerebral radiation necrosis by bevacizumab. J Clin Oncol 29:e159–e162
Mong S, Ellingson BM, Nghiemphu PL et al (2012) Persistent diffusion-restricted lesions in bevacizumab-treated malignant gliomas are associated with improved survival compared with matched controls. Am J Neuroradiol (AJNR) 33:1763–1770
Rieger J, Bahr O, Muller K et al (2010) Bevacizumab-induced diffusion-restricted lesions in malignant glioma patients. J Neurooncol 99:49–56
Kono K, Inoue Y, Nakayama K et al (2001) The role of diffusion-weighted imaging in patients with brain tumors. Am J Neuroradiol (AJNR) 22:1081–1088
Sugahara T, Korogi Y, Kochi M et al (1999) Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 9:53–60
White NS, Leergaard TB, D’Arceuil H et al (2013) Probing tissue microstructure with restriction spectrum imaging: histological and theoretical validation. Hum Brain Mapp 34:327–346
White NS, McDonald CR, Farid N et al (2013) Improved conspicuity and delineation of primary and metastatic brain tumors using “restriction spectrum imaging”: quantitative comparison with high b-value DWI and ADC. Am J Neuroradiol (AJNR) 34:958–964
Kothari PD, White NS, Farid N et al (2013) Longitudinal restriction spectrum imaging is resistant to pseudoresponse in patients with high-grade gliomas treated with bevacizumab. Am J Neuroradiol (AJNR) 34:1752–1757
Holland D, Kuperman JM, Dale AM (2010) Efficient correction of inhomogeneous static magnetic field-induced distortion in echo planar imaging. Neuroimage 50:175–183
Emblem KE, Bjornerud A (2009) An automatic procedure for normalization of cerebral blood volume maps in dynamic susceptibility contrast-based glioma imaging. Am J Neuroradiol (AJNR) 30:1929–1932
Boothe D, Young R, Yamada Y et al (2013) Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol 15:1257–1263
Sadraei NH, Dahiya S, Chao ST et al (2013) Treatment of cerebral radiation necrosis with bevacizumab: the Cleveland clinic experience. Am J Clin Oncol 90(3):192–200
Ellingson BM, Malkin MG, Rand SD et al (2010) Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging 31:538–548
Shah R, Vattoth S, Jacob R et al (2012) Radiation necrosis in the brain: imaging features and differentiation from tumor recurrence. Radiographics 32:1343–1359
Yoshii Y (2008) Pathological review of late cerebral radionecrosis. Brain Tumor Pathol 25:51–58
Acknowledgments
Funding: NIH Grants R01NS065838 (C.R.M.), RC2 DA29475 (A.M.D.) and EB00790-06 (A.M.D.) and 3P30CA023100-25S8 (S.K.), CAPES for PhD student scholarship (D.B.A.F).
Conflict of interest
None of the authors have any personal or institutional financial interest in drugs, materials, or devices described in this submission.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farid, N., Almeida-Freitas, D.B., White, N.S. et al. Combining diffusion and perfusion differentiates tumor from bevacizumab-related imaging abnormality (bria). J Neurooncol 120, 539–546 (2014). https://doi.org/10.1007/s11060-014-1583-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1583-2