Summary
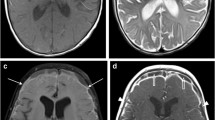
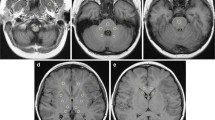
Seventy-nine patients with known or suspected central nervous system lesions were studied with MRI in a phase III double-blind study. Forty were given gadopentetate dimeglumine (Gd-DTPA) and 39 gadodiamide injection (Gd-DTPA BMA), a new low-osmolar nonionic contrast enhancing medium. The dosage was 0.1 mmol/kg body weight, corresponding to 0.2 ml/kg. Spin-echo sequences were performed before and immediately after injection. The safety and efficacy of the two contrast media were assessed. No changes were observed in blood pressure, heart rate or neurological status. Five adverse effects (two episodes of headaches, two of nausea and one of dizziness) were reported by 2 patients who received gadodiamide injection and 1 who received gadopentetate dimeglumine. All events were mild and their relationship to the contrast media was uncertain. For both contrast media statistically significant changes in serum iron were observed 24 h after injection. More than 70% of the patients had abnormal findings on MRI, and in 56% of these contrast enhancement of the abnormal structure or lesion was seen. Contrast enhancement provided the diagnosis in about 50%, changed it in 40% and increased diagnostic confidence in 95%.
Similar content being viewed by others
References
Valk J (1987) Contrast enhanced magnetic resonance imaging of the brain using Gadolinium-DTPA. Acta Radiol 28:659–665
Van Wagoner M, O'Toole M, Worah D, Leese PT, Quay SC (1991) A phase I clinical trial with gadodiamide injection, a nonionic MRI enhancement agent. Invest Radiol 26:980–986
Greco A, McNamara MT, Lanthiez P, Quay SC, Michelozzi G (1990) Gadodiamide injection: nonionic gadolinium chelate for MR imaging of the brain and spine—phase II–III clinical trials. Radiology 176:451–456
Sze G, Brant-Zawadzki M, Haughton VM, et al. (1992) Multicenter study of gadodiamide injection as a contrast enhancing agent in MR imaging of the brain and spine. Radiology (in press)
Van Wagoner M, O'Toole M, Quay SC (1990) Nonionic magnetic resonance imaging contrast agents. Clinical trial experience of safety, tolerance, and efficacy of gadodiamide injection. Invest Radiol 25:39–41
Spilker B (1984) Guide to clinical studies developing protocols. Raven Press, New York, p 162
Goldstein HA, Kashanian FK, Blumetti RF, Holyoak WL, Hugo FP, Blumenfield DM (1990) Safety assessment of gadopentetate dimeglumine in U.S. clinical trials. Radiology 174:17–23
Niendorf HP, Dinger JC, Haustein J, Cornelius I, Alhassan A, Clauss W (1991) Tolerance data of Gd-DTPA: a review. Eur J Radiol 13:15–20
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Valk, J., Algra, P.R., Hazenberg, C.J. et al. A double-blind, comparative study of gadodiamide injection and gadopentetate dimeglumine in MRI of the central nervous system. Neuroradiology 35, 173–177 (1993). https://doi.org/10.1007/BF00588486
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00588486