Summary
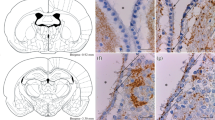
The circulation of the cerebrospinal fluid along the central canal and its access to the parenchyma of the spinal cord of the rat have been analyzed by injection of horseradish peroxidase (HRP) into the lateral ventricle. Peroxidase was found throughout the central canal 13 min after injection, suggesting a rapid circulation of cerebrospinal fluid along the central canal of the rat spinal cord. It was cleared from the central canal within 2 h, in contrast with the situation in the brain tissue, where it remained in the periventricular areas for 4 h. In the central canal, HRP bound to Reissner's fiber and the luminal surface of the ependymal cells; it penetrated through the intercellular space of the ependymal lining, reached the subependymal neuropil, the basement membrane of local capillaries, and appeared in the lumen of endothelial pinocytotic vesicles. Furthermore, it accumulated in the labyrinths of the basement membrane contacting the basolateral aspect of the ependymal cells. In ependymocytes, HRP was found in single pinocytotic vesicles. The blood vessels supplying the spinal cord were classified into two types. Type-A vessels penetrated the spinal cord laterally and dorsally and displayed the tracer along their external wall as far as the gray matter. Type-B vessels intruded into the spinal cord from the medial ventral sulcus and occupied the anterior commissure of the gray matter, approaching the central canal. They represented the only vessels marked by HRP along their course through the gray matter. HRP spread from the wall of type-B vessels, labeling the labyrinths, the intercellular space of the ependymal lining, and the lumen of the central canal. This suggests a communication between the central canal and the outer cerebrospinal fluid space, at the level of the medial ventral sulcus, via the intercellular spaces, the perivascular basement membrane and its labyrinthine extensions.
Similar content being viewed by others
References
Booz KH, Desaga U, Felsing T, Franz H, Stark M (1972) Subund interependymale Basalmembranlabyrinthe am Zentralkanal der weißen Ratte. Z Zellforsch 132:217–229
Borison HL, Borison R, McCarthy LE (1980) Brain stem penetration by horseradish peroxidase from the cerebrospinal fluid spaces in the cat. Exp Neurol 69:271–289
Bradbury MWB, Lathem W (1965) A flow of cerebrospinal fluid along the central canal of the spinal cord of the rabbit and communications between this canal and the sacral subarachnoid space. J Physiol (Lond) 181:785–800
Brightman MW (1965) The distribution within the brain of ferritin injected into cerebrospinal fluid compartments. I Ependymal distribution. J Cell Biol 26:99–123
Brightman MW, Prescott L, Reese TS (1975) Intercellular junctions of special ependyma. In: Knigge KM, Scott DE, Kobayashi H and Ishii S (eds) Brain endocrine interaction. II. The ventricular system in neuroendocrine mechanisms. Karger, Basel, pp 146–165
Burkholder PM (1974) Atlas of human glomerular pathology. Harper and Row, New York London, p 121
Cserr HF (1988) Role of secretion and bulk flow of brain interstitial fluid in brain volume regulation. Ann NY Acad Sci 529:9–20
Davson H, Welch K, Segal MB (1987) The physiology and pathophysiology of the cerebrospinal fluid. Churchill Livinstone, New York
Desaga U (1972) Form und Verteilung subependymaler Basalmembranlabyrinthe am Ventrikelsystem der Ratte. Z Zellforsch 132:553–562
Eisenberg HM, McLennan JE, Welch K, Treves S (1974a) Radioisotope ventriculography in cats with kaolin-induced hydrocephalus. Radiology 110:399–402
Eisenberg HM, McLennan JE, Welch K (1974b) Ventricular perfusion in cats with kaolin-induced hydrocephalus. J Neurosurg 41:20–28
Graham RC, Karnovsky M (1966) The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem 14:291–302
Hill JR (1957) The influence of drugs on ciliary activity. J Physiol (Lond) 139:157–166
Junqueira LCU, Bignolas G, Mourao PAS, Bonetti SS (1980) Quantitation of collagen-proteoglycan interaction in tissue sections. Connect Tissue Res 7:91–96
Krisch B, Leonhardt H, Oksche A (1984) Compartments and perivascular arrangement of the meninges covering the cerebral cortex of the rat. Cell Tissue Res 238:459–474
Leonhardt H (1972) Über die topographische Verteilung der subependymalen Basalmembranlabyrinthe im Ventrikelsystem des Kaninchengehirns. Z Zellforsch 127:392–406
Matakas F, Stechele S, Keller F (1978) Microcirculation within the cerebral extracellular space. In: Cervós-Navarro J, Betz E, Ebhardt G, Ferszt R, Wüllenweber R (eds) Advances in neurology, vol 20. Pathology of cerebrospinal microcirculation. Raven Press, New York, pp 125–131
Nakayama Y (1976) The openings of the central canal in the filum terminale internum of some mammals. J Neurocytol 5:531–544
Nakayama Y, Kohno K (1974) Number and polarity of the ependymal cilia in the central canal of some vertebrates. J Neurocytol 3:449–458
Rall DP, Oppelt WW, Patlak CS (1962) Extracellular space of brain as determined by diffusion of insulin from the ventricular system. Life Sci 1:43–48
Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA (1985) Evidence for a “paravascular” fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subaracnoid space. Brain Res 326:47–63
Rodríguez EM, Oksche A, Hein S, Yulis CR (1992) Cell biology of the subcommissural organ. Int Rev Cytol 135:39–121
Schubert P, Kreutzberg GW (1976) Communication between the neuron and the vessels. In: Cervós-Navarro J, Betz E, Matakas F, Wüllenweber R (eds) The cerebral vessel wall. Raven Press, New York, pp 207–213
Wagner HJ, Pilgrim C, Brandl J (1974) Penetration and removal of horseradish peroxidase injected into the cerebrospinal fluid: role of cerebral perivascular spaces, endothelium and microglia. Acta Neuropathol 27:299–315
Wood JH (1983) Physiology, pharmacology and dynamics of cerebrospinal fluid. In: Wood JK (ed) Neurobiology of cerebrospinal fluids. Plenum, New York, pp 1–16
Zervas NT, Liszczak TM, Mayberg MR, Black PMcL (1982) Cerebrospinal fluid may nourish cerebral vessels through pathways in the adventitia that may be analogous to systemic vasa vasorum. J Neurosurg 56:475–481
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cifuentes, M., Fernández-LLebrez, P., Pérez, J. et al. Distribution of intraventricularly injected horseradish peroxidase in cerebrospinal fluid compartments of the rat spinal cord. Cell Tissue Res. 270, 485–494 (1992). https://doi.org/10.1007/BF00645050
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00645050