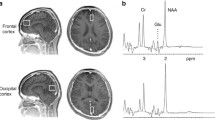
Abstract
We compared in vitro1H magnetic resonance spectroscopy (MRS) measurements of rat brain extracts (rats: 2–56 days old) with chromatographic measurements and in a further step also with results of in vivo MRS. The following substances can be reliably measured in brain extracts by in vitro MRS: N-acetylaspartate (NAA), total creatine (Cr), phosphorylethanoloamine (PE), taurine (Tau), glutamate (Glu), glutamine (Gln), γ-aminobutyrate (GABA) and alanine (Ala). Two different methods of MRS data evaluation compared with chromatographic data on Cr and NAA are shown. During development of the rat from day 2–56 brain concentrations of PE, Tau and Ala decrease, those of NAA, Cr, Glu and Gln increase, while GABA does not change. The developmental patterns of these substances are the same, whether measured by in vitro MRS or by chromatographic methods. Quantification of NAA, Cr, Tau, GABA and PE leads to the same results with both methods, while Glu, Gln and Ala concentrations determined by in vitro MRS are apparently lower than those measured chemically. The NAA/Cr ratios of 7 to 35-day-old rats were determined by in vivo1H MRS. These results correlate with chromatographic and in vitro data. Using appropriate methods in the in vivo and in vitro MR-technique, the obtained data compare well with the chromatographic results.
Similar content being viewed by others
References
Petroff, O. A. C. 1988. Biological1H-NMR spectroscopy. Comp. Biochem. Physiol. 90B 249–260.
Arus, C., Yen-Chang, and Barany, M. 1985. Proton nuclear magnetic resonance spectra of excised rat brain. Physiol. Chem. Phys. Med. NMR 17:23–33.
Marinier, D. L. S., Brignet, A., and Delmau, J. 1985. Perspectives d'emploi de la R.M.N pour l'étude biochimique de la substance blanche cerebrale. Arch. Int. Physiol. Biochim. 93:129–140.
Middlehurst, C. R., Beilharz, G. R., Hunt, G. E., Kuchel, P. W., and Johnson, G. F.-S. 1984. Proton nuclear magnetic resonance spectroscopy of rabbit brain homogenate. J. Neurochem. 42:878–879.
Ogino, T., Behar, K. L., and Shulman, R. G. 1986. Assignment of resonances in the1H-spectrum of rat brain in vitro and in situ by 2-D shift correlated and J-resolved spectroscopy. Abstracts Soc. Magn. Reson. Med. 3:985–986.
Young, R. S. K., Petroff, O. A. C., Dunham, S. L., and Cowan B. E. 1987. Neurotransmitter activity during prolonged and brief neonatal seizure. Pediatric Research 21:499A.
Young, R. S. K., and Cowan, B. E., Petroff, O. A. C., Novotny, E., Dunham, S. L., and Briggs, R. W. 1987. In vivo and in vitro1H-NMR study of hypoglycaemia during neonatal seizure. Ann. Neuro. 22:622–628.
Young, R. S. K., Petroff, O. A. C., Dunham, S. L., and Cowan B. E. 1987. What metabolic fuel is utilized by neonatal brain when hypoglycaemia complicates seizure? A1H-NMR study. Neurology 37:345.
Ackermann, J. J. H., Grove, T. H., Wong, G. G., Gadian, D. G., and Radda, G. K. 1980. Mapping of metabolites in whole animals by31P NMR using surface coils. Nature 283:167–170.
Barany, M., Spigos, D. G., Mok, E., Venkatasubramanian, P. N., Wilbur, A. C., and Langer, B. G. 1987. Magnetic Resonance Imaging 5:393–398.
Behar, K. L., den Hollander, J. A., Stromski, M. E., Ogino, T., Shulman, R. G., Petroff, O. A. C., and Prichard, J. W. 1983. High-resolution1H nuclear magnetic resonance study of cerebral hypoxia in vivo. Proc. natn. Acad. Sci. 80:4945–4948.
Bottomly, P. A., Edelstein, W. A., Foster, T. H., and Adams, W. A. 1985. In vivo solvent-suppressed localized hydrogen nuclear magnetic resonance spectroscopy: A window to metabolism? Proc. natn. Acad. Sci. 82:2148–2152.
Frahm, J., Bruhn, H., Gyngell, M. L., Merboldt, K. D., Hänicke, W., and Sauter, R. 1989. Localized high-resolution proton NMR spectroscopy using stimulated echoes: initial applications to human brain in vivo. Magnetic Resonance in Medicine 9:79–93.
Petroff, O. A. C., Prichard, J. W., Behar, K. L., Rothman, D., Alger, J. R., and Shulman, R. G. 1984. In vivo phosphorus nuclear magnetic resonance spectroscopy in status eptilepticus. Ann. Neurol. 16:169–177.
Petroff, O. A. C., Prichard, J. W., Behar, K. L., Rothman, D., Alger, J. R., and Shulman, R. G. 1985. Cerebral metabolism in hyper and hypocarbia:31P and1H NMR studies. Neurology 35:1681–1688.
Prichard, J. W., Alger, J. R., Behar, K. L., Petroff, O. A. C., and Shulman, R. G., 1985. Cerebral metabolic studies in vivo by31P NMR. Proc. natn. Acad. Sci. 80:2748–2751.
Prichard, J. W., and Shulman, R. G. 1986. NMR spectroscopy of brain metabolism in vivo. A. Rev. Neurosvci. 9:61–85.
Rothman, D. L., Behar, K. L., Hetherington, H. P., and Shulman, R. G. 1984. Homonuclear1H doubleresonance difference spectroscopy of the rat brain in vivo. Proc. natn. Acad. Sci. 81:6330–6334.
Young, R. S. K., Chen, B., Petroff, O. A. C., Gore, J. C., Cowan, B. E., Novotny, E. J., Wong, M., and -zuckerman, K. 1989. The effect of diazepam on neonatal seizure: In vivo31P and1H NMR study. Pediatric Research 25:27–31.
Cerdan, S., Parrilla, R., Santoro, J., and Rico, M. 1985.1H NMR detection of cerebral myo-inositol. FEBS Letters 187:165–172.
Fan, T. W.-M., Higashi, R. M., Lane, A. N., and Jardetzky, O. 1986. Combined use of1H-NMR and GC-MS for metabolite monitoring and in vivo1H-NMR assignments. Biochimica and Biophysica Acta 882:154–167.
Juengling, E., and Kammermeier, H. 1980. Rapid assay of adenine nucleotides or creatine compounds in extracts of cardiac tissue by paired-ion reverse-phase high-performance liquid chromatography. Analytical Biochem. 102:358–361.
Koller, K. J., Zaczek, R., and Coyle, J. T. 1984. N-acetyl-aspartyl-glutamate: Regional levels in rat brain and the effect of brain lesions as determined by a new HPLC method. J. Neurochem. 43:1136–1142.
Honegger, C. G., Burri, R., Langemann, H., and Kempf, A. 1984. Determination of neurotransmitter systems in human cerebrospinal fluid and rat nervous tissue by high-performance liquid chromatography with on-line data evaluation. J. Chromatography 309:53–61.
Bachmann, C., and Colombo, J.-P. 1983. Increased tryptophan uptake into the brain in hyperammonemia. Life Sci. 33:2417–2424.
Burri, R., Lazeyras, F., Aue, W. P., Straehl, P., Bigler, P., Althaus, U., and Herschkowitz, N. 1988. Correlation between31P NMR phosphomonoester and biochemically determined phosphorylethanolamine and phosphatidylethanolamine during development of the rat brain. Dev. Neurosci. 10:213–221.
Hore, P. J. 1983. Sovent suppression in Fourier transform nuclear magnetic resonance. J. Magn. Res. 55:283–300.
Campbell, I. D. Dobson, C. M., Williams, R. J. P., and Xavier, A. V. 1973. Resolution enhancement of protein PMR spectra using the difference between a broadened and a normal spectrum. J. Magn. Res. 11:172–183.
Agrawal, H. C., Davis, J. M., and Himwich, W.A. 1966. Postnatal changes in free amino acids pool of rat brain. J. Neurochem. 13:607–615.
Birken, D. L., and Oldendorf, W. H. 1989. N-acetyl-L-aspartic acid: A literature review of a compound prominent in1H-NMR spectroscopic studies of brain. Neuroscience and Biobehavioral Reviews 13:23–31.
Miyake, M., and Kakimoto, Y. 1981. Developmental changes of N-acety-L-glutamic acid in different brain regions and spinal cords of rat and guinea pigs. J. Neurochem. 37:1064–1067.
Koller, K. J., and Coyle, J. T. 1984. Ontogenesis of N-acetylaspartate and N-acetyl-aspartyl-glutamate in rat brain. Dev. Brain Res. 15:137–140.
van Zijl, P. C. M., Moonen, C. T. W., Alger, J. R., Cohen, J. S., and Chesnick S. A. 1989. High field localized proton spectroscopy in small volumes: Greatly improve localization and shimming using shielded strong gradients. Magnetic Resonance in Medicine:10:256–265.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Burri, R., Bigler, P., Straehl, P. et al. Brain development:1H magnetic resonance spectroscopy of rat brain extracts compared with chromatographic methods. Neurochem Res 15, 1009–1016 (1990). https://doi.org/10.1007/BF00965747
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00965747