Abstract
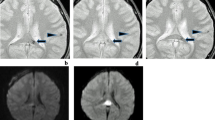
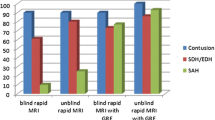
Gradient-echo (GE) MRI has been demonstrated to be the most sensitive current technique for detection of intracerebral haemosiderin, especially in the chronic stage of haemorrhage. Our purpose was to see whether GE MRI shows old haemorrhage indefinitely. We reviewed serial GE images of 105 adults with imaging features consistent with post-traumatic intracerebral haemorrhage, who had serial MRI at 1, 4–6, 12, and 24 months after trauma. Of 1235 scattered low-signal foci consistent with isolated intracerebral haemosiderin deposits on images at 4–6 months, 248 (20.1%) were not seen at 24-month assessment. Reviewing individual patients, we saw that in 71.8% of those with scattered haemosiderin deposits and 46.4% of those with haemosiderin surrounded by gliosis, the low-signal foci appeared less conspicuous with time. Even given certain limitations to the interpretation of these findings, it would appear that, even with the use of GE MRI, time affects the visibility of haemorrhagic intracerebral lesions. We therefore conclude that a time of 4–6 months to 1 year or slightly more should be recommended for most precise detection of haemosiderin deposits on MRI of head-injured patients, should this be thought desirable. Normal GE images may not exclude old haemorrhage.





Similar content being viewed by others
References
Gomori JM, Grossman RI, Goldberg HI, Zimmerman RA, Bilaniuk LT (1985) Intracranial hematomas: imaging by high-field MR. Radiology 157: 87–93
Zimmermann RA, Bilaniuk LT, Hackney DB, Goldberg HI, Grossman RI (1986) Head injury: early results of comparing CT and high-field MR. AJNR 7: 757–764
Hesselink JR, Dowd CF, Healy ME, Hajek P, Baker LL, Luerssen TG (1988) MR imaging of brain contusions: a comparative study with CT. AJNR 9: 269–278
Bradley WG Jr (1993) MR appearance of hemorrhage in the brain. Radiology 189: 15–26
Wardlaw JM, Statham PFX (2000) How often is haemosiderin not visible on routine MRI following traumatic intracerebral haemorrhage? Neuroradiology 42: 81–84
Atlas SW, Mark AS, Grossman RI, Gomori JM (1988) Intracranial hemorrhage: gradient-echo MR imaging at 1.5 T: comparison with spin-echo imaging and clinical applications. Radiology 168: 803–807
Unger EC, Cohen MS, Brown TR (1989) Gradient-echo imaging of hemorrhage at 1.5 Tesla. Magn Reson Imaging 7: 163–172
Gentry LR (1994) Imaging of closed head injury. Radiology 191: 1-17
Mittl RL, Grossman RI, Hiehle JF, Hurst RW, Gennarelli TA, Alburger GW (1994) Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. AJNR 15: 1583–1589
Patel MR, Edelman RR, Warach S (1996) Detection of hyperacute primary intraparenchymal hemorrhage by magnetic resonance imaging. Stroke 27: 2321–2324
Pellicano G, Beccani D, Cellerini M, Nistri M, Dal Pozzo G (1997) MRI evaluation of complications and long term sequelae of cranial trauma. Neuroradiology 39: S82
Parizel PM, Özsarlak Ö, van Goethem JW, et al (1998) Imaging findings in diffuse axonal injury after closed head trauma. Eur Radiol 8: 960–965
Bešenski N (2002) Traumatic injuries: imaging of head injuries. Eur Radiol 12: 1237–1252
Scharf J, Bräuherr E, Forsting M, Sartor K (1994) Significance of haemorrhagic lacunes on MRI in patients with hypertensive cerebrovascular disease and intracerebral haemorrhage. Neuroradiology 36: 504–508
Chan S, Kartha K, Yoon SS, Desmond DW, Hilal SK (1996) Multifocal hypointense cerebral lesions on gradient-echo MR are associated with chronic hypertension. AJNR 17: 1821–1827
Greenberg SM, Finkelstein SP, Schaefer PW (1996) Petechial hemorrhages accompanying lobar hemorrhage: detection by gradient-echo MRI. Neurology 46: 1751–1754
Offenbacher H, Fazekas F, Schmidt R, Koch M, Fazekas G, Kapeller P (1996) MR of cerebral abnormalities concomitant with primary intracerebral hematomas. AJNR 17: 573–578
Fazekas F, Kleinert R, Roob G, et al (1999) Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR 20: 637–642
Greenberg SM, O’Donnell HC, Schaefer PW, Kraft E (1999) MRI detection of new hemorrhages: potential marker of progression in cerebral amyloid angiopathy. Neurology 53: 1135–1138
Roob G, Schmidt R, Kapeller P, Lechner A, Hartung HP, Fazekas F (1999) MRI evidence of past cerebral microbleeds in a healthy elderly population. Neurology 52: 991–994
Kuzma BB, Goodman JM (2000) Improved identification of axonal shear injuries with gradient echo MR technique. Surg Neurol 53: 400–402
Roob G, Fazekas F (2000) Magnetic resonance imaging of cerebral microbleeds. Curr Opin Neurol 13: 69–73
Tsushima Y, Tamura T, Unno Y, Kusano S, Endo K (2000) Multifocal low-signal brain lesions on T2*-weighted gradient-echo imaging. Neuroradiology 42: 499–504
Tsushima Y, Tanizaki Y, Aoki J, Endo K (2002) MR detection of microhemorrhages in neurologically healthy adults. Neuroradiology 44: 31–36
Angeleri F, Majkowski J, Cacchiò G, et al (1999) Posttraumatic epilepsy risk factors: one-year prospective study after head injury. Epilepsia 40: 1222–1230
Campeau NG, Port JD, Miller GM (2001) Superiority of gradient echo imaging over echo-planar diffusion weighted imaging for the detection of hemosiderin. Neuroradiology 43: S89–S90
Grossman RI, Gomori JM, Goldberg HI, et al (1988) MR imaging of hemorrhagic conditions of the head and neck. Radiographics 8: 441–454
Fobben ES, Grossman RI, Atlas SW, et al (1989) MR characteristics of subdural hematomas and hygromas at 1.5 T. AJNR 10: 687–693
Zyed A, Hayman LA, Bryan RN (1991) MR imaging of intracerebral blood: diversity in the temporal pattern at 0.5 and 1.0 T. AJNR 12: 469–474
Thulborn KR, Sorensen AG, Kowall NW, et al (1990) The role of ferritin and hemosiderin in the MR appearance of cerebral hemorrhage: a histopathologic biochemical study in rats. AJNR 11: 291–297
Chen JC, Hardy PA, Kucharczyk W, et al (1993) MR of human postmortem brain tissue: correlative study between T2 and assays of iron and ferritin in Parkinson and Huntington disease. AJNR 14: 275–281
Chen JC, Hardy PA, Clauberg M, et al (1989) T2 values in the human brain: comparison with quantitative assays of iron and ferritin. Radiology 173: 521–526
Vymazal J, Urgosik D, Bulte JW (2000) Differentiation between hemosiderin- and ferritin-bound brain iron using NMR and MRI. Cell Mol Biol 46: 835–842
Darrow VC, Alvord EC Jr, Mack LA, Hodson WA (1988) Histologic evolution of the reactions to hemorrhage in the premature human infant’s brain. A combined ultrasound and autopsy study and a comparison with the reaction in adults. Am J Pathol 130: 44–58
Acknowledgements
We thank Professor F. Angeleri, lately Director of the Neurological Clinic of the General Hospital and University of Ancona, Professor M. Signorino, Dr G. Cacchiò, Dr S. D’Acunto and their colleagues for their work on prospective study on TBI. We are especially grateful to Dr L. Regnicolo, and to M. Cola, C. Novelli and T. Tarabelli of the MRI Unit of the University of Ancona, who made the long-term imaging study possible. We also thank Dr Simone Salvolini of the University of Ancona, for his assistance with data collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Messori, A., Polonara, G., Mabiglia, C. et al. Is haemosiderin visible indefinitely on gradient-echo MRI following traumatic intracerebral haemorrhage?. Neuroradiology 45, 881–886 (2003). https://doi.org/10.1007/s00234-003-1048-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-003-1048-3