Abstract
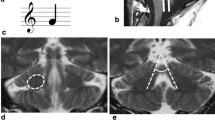
The aim of the present study was to demonstrate characteristic signal changes of the pontine base on T2-weighted images of patients with SCA 1, and to elucidate the relationship between abnormal high-intensities of the pontine base on T2-weighted images and the findings on multishot diffusion-weighted images. We assessed abnormal signals of the pontine base on T2-weighted images from 50 controls and six patients with SCA 1 diagnosed by genetic analysis. At the same time, we evaluated the degeneration of the transverse pontine fibers in the pontine base by multishot diffusion-weighted imaging. A midline high-intensity was seen in the pontine base on T2-weighted images in two of the 50 controls and five of the six patients with SCA 1. The midline high-intensity had a sensitivity of 83.3% for patients and a specificity of 96.0% for controls. Multishot diffusion-weighted imaging demonstrated the degeneration—the amorphous-pattern signal—of the transverse pontine fibers in four (66.7%) of the six patients. In the other two patients, the zebra-pattern signal was seen in the pontine base. The midline high-intensity on T2-weighted images appears to be one of characteristic MRI findings of SCA 1. Multishot diffusion-weighted imaging suggested that the midline high-intensity should reflect the degeneration of the transverse pontine fibers.




Similar content being viewed by others
References
Orr HT, Chung MY, Banfi S et al (1993) Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet 4:221–226
Takiyama Y, Nishizawa M, Tanaka H et al (1993) The gene for Machado-Joseph disease maps to human chromosome 14q. Nat Genet 4:300–304
Koide R, Ikeuchi T, Onodera O et al (1994) Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA). Nat Genet 6:9–13
Nagafuchi S, Yanagisawa H, Sato K et al (1996) Dentatorubral and pallidoluysian atrophy expansion of an unstable CAG trinucleotide on chromosome 12p. Nat Genet 6:14–18
Matsuyama Z, Kawakami H, Maruyama H et al (1997) Molecular features of the CAG repeats of spinocerebellar ataxia 6 (SCA6). Hum Mol Genet 6:1283–1288
Ishikawa K, Tanaka H, Saito M et al (1997) Japanese families with autosomal dominant pure cerebellar ataxia map to chromosome 19p13.1–p13.2 and are strongly associated with mild CAG expansions in the spinocerebellar ataxia type-6 gene in chromosome 19p13.1. Am J Hum Genet 61:336–346
Wenning GK, Ben Shlomo Y, Magalhaes M, Daniel SE, Quinn NP (1994) Clinical features and natural history of multiple system atrophy; an analysis of 100 cases. Brain 117:835–845
Gilman S, Low P, Quinn N et al (1998) Consensus statement on the diagnosis of multiple system atrophy. Clin Auton Res 8:359–362
Savoiardo M, Strada L, Girotti F et al. (1990) Olivopontocerebellar atrophy: MR diagnosis and relationship to multisystem atrophy. Radiology 174:693–696
Idezuka J, Onodera O, Yuasa T, Tsuji S, Ito J (1993) MRI findings of olivopontocerebellar atrophy and Machado-Joseph disease: diagnostic value of transverse pontine fibers. Rinsho Shinkeigaku 33:289–293
Kojima S (1993) Clinical types of spinocerebellar degeneration and evaluation with MR imaging. Rinsho Shinkeigaku 33:1294–1296
Naka H, Ohshita T, Murata Y, Imon Y, Mimori Y, Nakamura S (2002) Characteristic MRI findings in multiple system atrophy: comparison of the three subtypes. Neuroradiology 44:204–209
Yagishita T, Kojima S, Hirayama K (1995) MRI study of degenerative process in multiple system atrophy. Rinsho Shinkeigaku 35:126–131
Nagaoka U, Suzuki Y, Kawanami T et al (1999) Regional differences in genetic subgroup frequency in hereditary cerebellar ataxia, and a morphometrical study of brain MR images in SCA1, MJD and SCA6. J Neurol Sci 164:187–194
Nakayama T, Nakayama K, Takahashi Y et al (2001) Case of spinocerebellar ataxia type 1 showing high intensity lesions in the frontal white matter on T2-weighted magnetic resonance images. Med Sci Monit 7:299–303
Klockgether T, Skalej M, Wedekind D et al (1998) Autosomal dominant cerebellar ataxia type I. MRI-based volumetry of posterior fossa structures and basal ganglia in spinocerebellar ataxia types 1, 2 and 3. Brain 121:1687–1693
Mascalchi M, Tosetti M, Plasmati R et al (1998) Proton magnetic resonance spectroscopy in an Italian family with spinocerebellar ataxia type 1. Ann Neurol 43:244–252
Burk K, Abele M, Fetter M et al (1996) Autosomal dominant cerebellar ataxia type I clinical features and MRI in families with SCA1, SCA2 and SCA3. Brain 119:1497–1505
Koide R, Onodera O, Ikeuchi T et al (1997) Atrophy of the cerebellum and brainstem in dentatorubral pallidoluysian atrophy: influence of CAG repeat size on MRI findings. Neurology 49:1605–1612
Otsuka H, Harada M, Hieda M et al (1996) Magnetic resonance studies of dentato-rubro-pallido-luysian atrophy-correlation with clinical severity and age of onset. No To Shinkei 48:818–823
Muñoz E, Milà M, Sánchez A et al (1999) Dentatorubropallidoluysian atrophy in a Spanish family: a clinical, radiological, pathological, and genetic study. J Neural Neurosurg Psychiatry 67:811–814
Uyama E, Kondo I, Uchino M et al (1995) Dentatorubral-pallidoluysian atrophy (DRPLA): clinical, genetic, and neuroradiologic studies in a family. J Neurol Sci 130:146–153
Adachi M, Hosoya T, Yamaguchi K, Kawanami T, Kato T (2000) Diffusion- and T2-weighted MRI of the transverse pontine fibres in spinocerebellar degeneration. Neuroradiology 42:803–809
Murata Y, Yamaguchi S, Kawakami H et al (1998) Characteristic magnetic resonance imaging findings in Machado-Joseph disease. Arch Neurol 55:33–37
Adachi M, Hosoya T, Haku T, Yamaguchi K, Kawanami T (1999) Evaluation of the substantia nigra in Patients with parkinsonian syndrome accomplished using multishot diffusion-weighted imaging. Am J Neuroradiol 20:1500–1506
Adachi M, Kawakatsu S, Hosoya T et al (2003) Morphology of the inner structure of the hippocampal formation in Alzheimer disease. Am J Neuroradiol 24:1575–1581
Gilman S, Sima AA, Junck L et al (1996) Spinocerebellar ataxia type 1 with multiple system degeneration and glial cytoplasmic inclusions. Ann Neurol 39:241–255
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adachi, M., Kawanami, T., Ohshima, H. et al. Characteristic signal changes in the pontine base on T2- and multishot diffusion-weighted images in spinocerebellar ataxia type 1. Neuroradiology 48, 8–13 (2006). https://doi.org/10.1007/s00234-005-0002-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-005-0002-y