Abstract
Introduction
The purpose of this study was to evaluate the clinical features and the characteristics of MR images of patients with end-stage hepatic failure.
Methods
We reviewed the MR findings and clinical features of 31 consecutive patients (20 men, 11 women=31, mean age 58.7 years) who had been diagnosed with clinical hepatic encephalopathy. Associations between the lesion locations on each MR sequence were analyzed using a binominal test. The clinical and MR findings were compared in relation to the etiology and clinical status.
Results
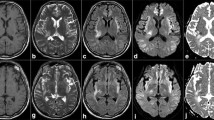
The most frequently involved site, seen as high signal intensity on T2-W images, was the corpus callosum (20 patients), followed by the dentate nucleus (16 patients) and the globus pallidus (13 patients). Significant associations were seen between the pallidus and the crus cerebri, between the crus cerebri and the red nucleus, between the crus cerebri and the dentate nucleus, and between the red nucleus and the dentate nucleus on the T2-W and DW images (P < 0.004). The crus cerebri, red nucleus, and dentate nucleus were involved concurrently with the corpus callosum more frequently in hepatic encephalopathy grades 3 and 4.
Conclusion
Concurrent involvement of the globus pallidus–crus cerebri–red nucleus–dentate nucleus axis was the main MR pattern in end-stage hepatic encephalopathy, which connected with various areas of the brain. We hypothesize that these overlapping MR features could be regarded as an entity denoted as the “hepatic encephalopathy continuum”.




Similar content being viewed by others
References
Hazell A, Butterworth R (1999) Hepatic encephalopathy: An update of pathophysiologic mechanisms. Proc Soc Exp Biol Med 222:99–112
Sherlock S (1977) Hepatic encephalopathy. Br J Hosp Med 17:144–146, 151–154, 159
Inoue E, Hori S, Narumi Y et al (1991) Portal-systemic encephalopathy: presence of basal ganglia lesions with high signal intensity on MR images. Radiology 179:551–555
Zeneroli M, Cioni G, Crisi G et al (1991) Globus pallidus alterations and brain atrophy in liver cirrhosis patients with encephalopathy: an MR imaging study. Magn Reson Imaging 9:295–302
Pujol A, Pujol J, Graus F et al (1993) Hyperintense globus pallidus on T1-weighted MRI in cirrhotic patients is associated with severity of liver failure. Neurology 43:65–69
Bernuau J, Rueff B, Benhamou JP (1986) Fulminant and subfulminant liver failure: definitions and causes. Semin Liver Dis 6:97–106
Hoofnagle JH, Carithers RL Jr, Shapiro C et al (1995) Fulminant hepatic failure: summary of a workshop. Hepatology 21:240–252
Kato M, Hughes R, Keays R et al (1992) Electron microscopic study of brain capillaries in cerebral edema from fulminant hepatic failure. Hepatology 15:1060–1066
Traber P, Dal Canto M, Ganger D et al (1987) Electron microscopic evaluation of brain edema in rabbits with galactosamine-induced fulminant hepatic failure: ultrastructure and integrity of the blood-brain barrier. Hepatology 7:1272–1277
Vymazal J, Babis M, Brooks RA et al (1996) T1 and T2 alterations in the brains of patients with hepatic cirrhosis. AJNR Am J Neuroradiol 17:333–336
Cordoba J, Raguer N, Flavia M et al (2003) T2 hyperintensity along the cortico-spinal tract in cirrhosis relates to functional abnormalities. Hepatology 38:1026–1033
Matsusue E, Kinoshita T, Ohama E et al (2005) Cerebral cortical and white matter lesions in chronic hepatic encephalopathy: MR-pathologic correlations. AJNR Am J Neuroradiol 26:347–351
Rovira A, Cordoba J, Sanpedro F et al (2002) Normalization of T2 signal abnormalities in hemispheric white matter with liver transplant. Neurology 59:335–341
Volk ML, Marrero JA (2006) Advances in critical care hepatology. Minerva Anestesiol 72:269–281
Trey C, Davidson CS (1970) The management of fulminant hepatic failure. Prog Liver Dis 3:282–298
Gill RQ, Sterling RK (2001) Acute liver failure. J Clin Gastroenterol 33:191–198
Giuffrida R, Aicardi G, Canedi A et al (1993) Excitatory amino acids as neurotransmitters of cortical and cerebellar projections to the red nucleus: an immunocytochemical study in the guinea pig. Somatosens Mot Res 10:365–376
Walberg F, Dietrichs E (1986) Is there a reciprocal connection between the red nucleus and the interposed cerebellar nuclei? Conclusions based on observations of anterograde and retrograde transport of peroxidase-labelled lectin in the same animal. Brain Res 397:73–85
Nieoullon A, Dusticier N (1981) Increased glutamate decarboxylase activity in the red nucleus of the adult cat after cerebellar lesions. Brain Res 224:129–139
Naus C, Flumerfelt B, Hrycyshyn A (1984) Topographic specificity of aberrant cerebellorubral projections following neonatal hemicerebellectomy in the rat. Brain Res 309:1–15
Norenberg M, Bender A (1994) Astrocyte swelling in liver failure: role of glutamine and benzodiazepines. Acta Neurochir Suppl (Wien) 60:24–27
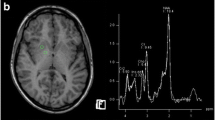
Taylor-Robinson SD, Sargentoni J, Oatridge A et al (1996) MR imaging and spectroscopy of the basal ganglia in chronic liver disease: correlation of T1-weighted contrast measurements with abnormalities in proton and phosphorus-31 MR spectra. Metab Brain Dis 11:249–268
Felipo V (2006) Contribution of altered signal transduction associated to glutamate receptors in brain to the neurological alterations of hepatic encephalopathy. World J Gastroenterol 12:7737–7743
Angaut P, Batini C, Billard J et al (1986) The cerebellorubral projection in the rat: retrograde anatomical study. Neurosci Lett 68:63–68
Nakamura Y, Kitao Y, Moriizumi T et al (1987) Electron microscopic study of the rubrocerebellar projection in the cat. J Comp Neurol 258:611–621
Caughell K, Flumerfelt B (1977) The organisation of the cerebellorubral projection: an experiment study in the rat. J Comp Neurol 176:295–306
Alvarez-Leal M, Contreras-Hernandez D, Chavez A et al (2001) Leukocyte arylsulfatase A activity in patients with alcohol-related cirrhosis. Am J Hum Biol 13:297–300
Rovira A, Mínguez B, Aymerich FX et al (2007) Decreased white matter lesion volume and improved cognitive function after liver transplantation. Hepatology 46:1485–1490
Regan M, Huang Y, Kim Y et al (2007) Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J Neurosci 27:6607–6619
Sajith J, Ditchfield A, Katifi H (2006) Extrapontine myelinolysis presenting as acute parkinsonism. BMC Neurol 6:33
Sterns R, Riggs J, Schochet S (1986) Osmotic demyelination syndrome following correction of hyponatremia. N Engl J Med 314:1535–1542
Martin R (2004) Central pontine and extrapontine myelinolysis: the osmotic demyelination syndromes. J Neurol Neurosurg Psychiatry 75 [Suppl 3]:iii22–iii28
Adams R, Victor M, Mancall E (1959) Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry 81:154–172
Kleinschmidt-DeMasters B, Norenberg M (1981) Rapid correction of hyponatremia causes demyelination: relation to central pontine myelinolysis. Science 211:1068–1070
Butterworth R, Giguere J, Michaud J et al (1987) Ammonia: key factor in the pathogenesis of hepatic encephalopathy. Neurochem Pathol 6:1–12
Kreis R, Farrow N, Ross BD (1991) Localized 1H NMR spectroscopy in patients with chronic hepatic encephalopathy. Analysis of changes in cerebral glutamine, choline and inositols. NMR Biomed 4:109–116
Cordoba J, Gottstein J, Blei A (1996) Glutamine, myo-inositol, and organic brain osmolytes after portocaval anastomosis in the rat: implications for ammonia-induced brain edema. Hepatology 24:919–923
Shawcross D, Balata S, Olde Damink S et al (2004) Low myo-inositol and high glutamine levels in brain are associated with neuropsychological deterioration after induced hyperammonemia. Am J Physiol Gastrointest Liver Physiol 287:G503–G509
Silver S, Schroeder B, Sterns R et al (2006) Myoinositol administration improves survival and reduces myelinolysis after rapid correction of chronic hyponatremia in rats. J Neuropathol Exp Neurol 65:37–44
Victor M, Adams R, Cole M (1965) The acquired (non-Wilsonian) type of chronic hepatocerebral degeneration. Medicine (Baltimore) 44:345–396
Kleinschmidt-DeMasters B, Filley C, Rojiani A (2006) Overlapping features of extrapontine myelinolysis and acquired chronic (non-Wilsonian) hepatocerebral degeneration. Acta Neuropathol (Berl) 112:605–616
Lee J, Lacomis D, Comu S et al (1998) Acquired hepatocerebral degeneration: MR and pathologic findings. AJNR Am J Neuroradiol 19:485–487
Goebel H, Zur P (1972) Central pontine myelinolysis. A clinical and pathological study of 10 cases. Brain 95:495–504
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, H.K., Lee, H.J., Lee, W. et al. Pattern approach to MR imaging in patients with end-stage hepatic failure: a proposal for a new disease entity “hepatic encephalopathy continuum”. Neuroradiology 50, 683–691 (2008). https://doi.org/10.1007/s00234-008-0395-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-008-0395-5