Abstract
Background
Nusinersen, the only treatment approved by the United States Food and Drug Administration for spinal muscular atrophy (SMA), is delivered intrathecally. Many children with SMA have extensive spinal instrumentation and deformities, often precluding the use of standard approaches for gaining intrathecal access. Furthermore the anatomical distortion that often occurs with rotoscoliosis can complicate the use of fluoroscopic guidance. Compared to fluoroscopy, CT affords superior guidance for complex needle placements. This opens up alternatives to the posterior (interlaminar) technique, including transforaminal and caudal approaches.
Objective
This study describes the early results of technical success, complications and radiation dose of intrathecal delivery of nusinersen using cone-beam CT guidance with two-axis fluoroscopic navigational overlay.
Materials and methods
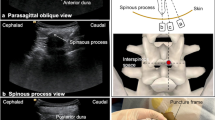
We conducted a retrospective review of 15 consecutive nusinersen injections performed in four children with SMA and extensive spinal hardware precluding standard posterior lumbar puncture techniques. These children were treated using transforaminal thecal access employing cone-beam CT with navigational overlay. We analyzed results including technical success, complications and total fluoroscopy time.
Results
All procedures were technically successful. No major complications and one minor complication were reported; the minor complication was a post-procedural neuropathic headache that was attributed to procedural positioning and was treated successfully with gabapentin. The average procedural fluoroscopy time and air kerma were 1.9 min and 55.8 mGy, respectively.
Conclusion
Cone-beam CT guidance with two-axis navigational overlay is a safe, effective method for gaining transforaminal intrathecal access in children with spinal abnormalities and hardware precluding the use of standard techniques.




Similar content being viewed by others
References
Lunn MR, Wang CH (2008) Spinal muscular atrophy. Lancet 371:2120–2133
Prior TW, Finanger E (2000) Spinal muscular atrophy. In: Pagon RA, Adam MP, Ardinger HH et al (eds) GeneReviews®. University of Washington, Seattle
Swoboda KJ, Prior TW, Scott CB et al (2005) Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann Neurol 57:704–712
Hua Y, Sahashi K, Hung G et al (2010) Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev 24:1634–1644
Hua Y, Vickers TA, Baker BF et al (2007) Enhancement of SMN2 exon 7 inclusion by antisense oligonucleotides targeting the exon. PLoS Biol 5:e73
Chiriboga CA, Swoboda KJ, Darras BT et al (2016) Results from a phase 1 study of nusinersen (ISIS-SMN(Rx)) in children with spinal muscular atrophy. Neurology 86:890–897
Finkel R, Kuntz N, Mercuri E et al (2017) Efficacy and safety of nusinersen in infants with spinal muscular atrophy (SMA): final results from the phase 3 ENDEAR study. Eur J Paediatr Neurol 21:e14–e15
Finkel RS, Chiriboga CA, Vajsar J et al (2016) Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet 388:3017–3026
Mercuri E, Finkel R, Kirschner J et al (2017) Interim analysis of the phase 3 CHERISH study evaluating nusinersen in patients with later-onset spinal muscular atrophy (SMA): primary and descriptive secondary endpoints. Eur J Paediatr Neurol 21:e15
U.S. Food and Drug Administration (2017) FDA approves first drug for spinal muscular atrophy. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm534611.htm. Accessed 28 Aug 2017
Hazra AK, Bhattacharya D, Mukherjee S et al (2016) Ultrasound versus fluoroscopy-guided caudal epidural steroid injection for the treatment of chronic low back pain with radiculopathy: a randomised, controlled clinical trial. Indian J Anaesth 60:388
Jones SB, Shaw DW, Jacobson LE (1997) A transsacral approach through the sacral hiatus for myelography. AJR Am J Roentgenol 169:1179–1181
Andreisek G, Jenni M, Klingler D et al (2013) Access routes and reported decision criteria for lumbar epidural drug injections: a systematic literature review. Skelet Radiol 42:1683–1692
Abi-Jaoudeh N, Venkatesan AM, Van der Sterren W et al (2015) Clinical experience with cone-beam CT navigation for tumor ablation. J Vasc Interv Radiol 26:214–219
Tselikas L, Joskin J, Roquet F et al (2015) Percutaneous bone biopsies: comparison between flat-panel cone-beam CT and CT-scan guidance. Cardiovasc Intervent Radiol 38:167–176
McKay T, Ingraham CR, Johnson GE et al (2016) Cone-beam CT with fluoroscopic overlay versus conventional CT guidance for percutaneous abdominopelvic abscess drain placement. J Vasc Interv Radiol 27:52–57
Perry BC, Monroe EJ, McKay T et al (2017) Pediatric percutaneous osteoid osteoma ablation: CONE-beam CT with fluoroscopic overlay versus conventional CT guidance. Cardiovasc Intervent Radiol. https://doi.org/10.1007/s00270-017-1685-2
Haché M, Swoboda KJ, Sethna N et al (2016) Intrathecal injections in children with spinal muscular atrophy: nusinersen clinical trial experience. J Child Neurol 31:899–906
Schwentker EP, Gibson DA (1976) The orthopaedic aspects of spinal muscular atrophy. J Bone Joint Surg 58:32–38
Phillips DP, Roye DP Jr, Farcy JP et al (1990) Surgical treatment of scoliosis in a spinal muscular atrophy population. Spine 15:942–945
Hong JH, Park EK, Park KB et al (2017) Comparison of clinical efficacy in epidural steroid injections through transforaminal or parasagittal approaches. Korean J Pain 30:220–228
Botwin KP, Gruber RD, Bouchlas CG et al (2000) Complications of fluoroscopically guided transforaminal lumbar epidural injections. Arch Phys Med Rehabil 81:1045–1050
Lutz GE, Vad VB, Wisneski RJ (1998) Fluoroscopic transforaminal lumbar epidural steroids: an outcome study. Arch Phys Med Rehabil 79:1362–1366
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
N. Natarajan discloses research funding from Biogen for execution of clinical trial projects. S.D. Apkon discloses consulting with Biogen. J.J. Weaver, D.W.W. Shaw, K.S.H. Koo, G.M. Shivaram and E.J. Monroe have no financial disclosures or conflicts of interest.
Rights and permissions
About this article
Cite this article
Weaver, J.J., Natarajan, N., Shaw, D.W.W. et al. Transforaminal intrathecal delivery of nusinersen using cone-beam computed tomography for children with spinal muscular atrophy and extensive surgical instrumentation: early results of technical success and safety. Pediatr Radiol 48, 392–397 (2018). https://doi.org/10.1007/s00247-017-4031-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-017-4031-6