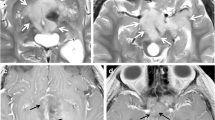
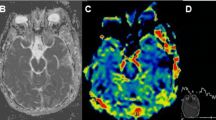
Abstract
Assessing tumor response is a large part of everyday clinical work in neuroradiology. However in the setting of tumor treatment, distinguishing tumor progression from treatment-related changes is difficult on conventional MRI sequences. This is made even more challenging in children where mainstay advanced imaging techniques that are often used to decipher progression versus treatment-related changes have technical limitations. In this review, we highlight the challenges in pediatric neuro-oncologic tumor assessment with discussion of pseudophenomenon including pseudoresponse and pseudoprogression. Additionally, we discuss the advanced imaging techniques often employed in neuroradiology to distinguish between pseudophenomenon and true progressive disease.











Similar content being viewed by others
References
Taal W, Brandsma D, de Bruin HG et al (2008) Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 113:405–410
Brandsma D, Stalpers L, Taal W et al (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9:453–461
Verma N, Cowperthwaite MC, Burnett MG, Markey MK (2013) Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro Oncol 15:515–534
Kreisl TN, Kim L, Moore KA et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745
Vredenburgh JJ, Desjardins A, Herndon JES et al (2007) Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 13:1253–1259
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Norden AD, Drappatz J, Muzikansky A et al (2009) An exploratory survival analysis of anti-angiogenic therapy for recurrent malignant glioma. J Neurooncol 92:149–155
Negretti L, Blanchard P, Couanet D et al (2012) Pseudoprogression after high-dose busulfan-thiotepa with autologous stem cell transplantation and radiation therapy in children with brain tumors: impact on survival. Neuro Oncol 14:1413–1421
Chassot A, Canale S, Varlet P et al (2012) Radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. J J Neurooncol 106:399–407
Ceschin R, Kurland BF, Abberbock SR et al (2015) Parametric response mapping of apparent diffusion coefficient as an imaging biomarker to distinguish pseudoprogression from true tumor progression in peptide-based vaccine therapy for pediatric diffuse intrinsic pontine glioma. AJNR Am J Neuroradiol 36:2170–2176
Prager AJ, Martinez N, Beal K et al (2015) Diffusion and perfusion MRI to differentiate treatment-related changes including pseudoprogression from recurrent tumors in high-grade gliomas with histopathologic evidence. AJNR Am J Neuroradiol 36:877–885
Hatzoglou V, Ulaner GA, Zhang Z et al (2013) Comparison of the effectiveness of MRI perfusion and fluorine-18 FDG PET-CT for differentiating radiation injury from viable brain tumor: a preliminary retrospective analysis with pathologic correlation in all patients. Clin Imaging 37:451–457
Shin KE, Ahn KJ, Choi HS et al (2014) DCE and DSC MR perfusion imaging in the differentiation of recurrent tumour from treatment-related changes in patients with glioma. Clin Radiol 69:e264–e272
Essig M, Shiroishi MS, Nguyen TB et al (2013) Perfusion MRI: the five most frequently asked technical questions. AJR Am J Roentgenol 200:24–34
Petrella JR, Provenzale JM (2000) MR perfusion imaging of the brain: techniques and applications. AJR Am J Roentgenol 175:207–219
Barajas RF, Chang JS, Sneed PK et al (2009) Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol 30:367–372
Barajas RF, Chang JS, Segal MR et al (2009) Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 253:486–496
Carceller F, Fowkes LA, Khabra KS et al (2016) Pseudoprogression in children, adolescents and young adults with non-brainstem high grade glioma and diffuse intrinsic pontine glioma. J Neurooncol 129:109–121
Gururangan S, Chi SN, Young Poussaint T et al (2010) Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: a pediatric brain tumor consortium study. J Clin Oncol 28:3069–3075
Gururangan S, Fangusaro J, Young Poussaint T et al (2012) Lack of efficacy of bevacizumab + irinotecan in cases of pediatric recurrent ependymoma — a pediatric brain tumor consortium study. Neuro Oncol 14:1404–1412
Thompson EM, Guillaume DJ, Dósa E et al (2012) Dual contrast perfusion MRI in a single imaging session for assessment of pediatric brain tumors. J Neurooncol 109:105–114
Kruser TJ, Mehta MP, Robins HI (2013) Pseudoprogression after glioma therapy: a comprehensive review. Expert Rev Neurother 13:389–403
Parkes LM, Rashid W, Chard DT, Tofts PS (2004) Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med 51:736–743
Patel P, Baradaran H, Delgado D et al (2017) MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: a systematic review and meta-analysis. Neuro Oncol 19:118–127
Yoo RE, Choi SH (2016) Recent application of advanced MR imaging to predict pseudoprogression in high-grade glioma patients. Magn Reson Med Sci 15:165–177
Welker K, Boxerman J, Kalnin A et al (2015) ASFNR recommendations for clinical performance of MR dynamic susceptibility contrast perfusion imaging of the brain. AJNR Am J Neuroradiol 36:E41–E51
Grade M, Hernandez Tamames JA, Pizzini FB et al (2015) A neuroradiologist's guide to arterial spin labeling MRI in clinical practice. Neuroradiology 57:1181–1202
Noguchi T, Yoshiura T, Hiwatashi A et al (2008) Perfusion imaging of brain tumors using arterial spin-labeling: correlation with histopathologic vascular density. AJNR Am J Neuroradiol 29:688–693
Yeom KW, Mitchell LA, Lober RM et al (2014) Arterial spin-labeled perfusion of pediatric brain tumors. AJNR Am J Neuroradiol 35:395–401
Choi YJ, Kim HS, Jahng GH et al (2013) Pseudoprogression in patients with glioblastoma: added value of arterial spin labeling to dynamic susceptibility contrast perfusion MR imaging. Acta Radiol 54:448–454
Guo AC, Cummings TJ, Dash RC, Provenzale JM (2002) Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology 224:177–183
Hayashida Y, Hirai T, Morishita SY et al (2006) Diffusion-weighted imaging of metastatic brain tumors: comparison with histologic type and tumor cellularity. AJNR Am J Neuroradiol 27:1419–1425
Herneth AM, Guccione S, Bednarski M (2003) Apparent diffusion coefficient: a quantitative parameter for in vivo tumor characterization. Eur J Radiol 45:208–213
Asao C, Korogi Y, Kitajima M et al (2005) Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am J Neuroradiol 26:1455–1460
Hein PA, Eskey CJ, Dunn JF, Hug EB (2004) Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol 25:201–209
Matsusue E, Fink JR, Rockhill JK et al (2010) Distinction between glioma progression and post-radiation change by combined physiologic MR imaging. Neuroradiology 52:297–306
Zeng QS, Li CF, Liu H et al (2007) Distinction between recurrent glioma and radiation injury using magnetic resonance spectroscopy in combination with diffusion-weighted imaging. Int J Radiat Oncol Biol Phys 68:151–158
Lee WJ, Choi SH, Park CKH et al (2012) Diffusion-weighted MR imaging for the differentiation of true progression from pseudoprogression following concomitant radiotherapy with temozolomide in patients with newly diagnosed high-grade gliomas. Acad Radiol 19:1353–1361
Zarifi M, Tzika AA (2016) Proton MRS imaging in pediatric brain tumors. Pediatr Radiol 46:952–962
Martín Noguerol T, Sánchez-González J, Martínez Barbero JP et al (2016) Clinical imaging of tumor metabolism with 1H magnetic resonance spectroscopy. Magn Reson Imaging Clin N Am 24:57–86
Smith EA, Carlos RC, Junck LR et al (2009) Developing a clinical decision model: MR spectroscopy to differentiate between recurrent tumor and radiation change in patients with new contrast-enhancing lesions. AJR Am J Roentgenol 192:W45–W52
Elias AE, Carlos RC, Smith EA et al (2011) MR spectroscopy using normalized and non-normalized metabolite ratios for differentiating recurrent brain tumor from radiation injury. Acad Radiol 18:1101–1108
Walecki J, Sokól M, Pieniazek P et al (1999) Role of short TE 1H-MR spectroscopy in monitoring of post-operation irradiated patients. Eur J Radiol 30:154–161
Dhermain FG, Hau P, Lanfermann H et al (2010) Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol 9:906–920
Alexander A, Murtha A, Abdulkarim B et al (2006) Prognostic significance of serial magnetic resonance spectroscopies over the course of radiation therapy for patients with malignant glioma. Clin Invest Med 29:301–311
Quon H, Brunet B, Alexander AW et al (2011) Changes in serial magnetic resonance spectroscopy predict outcome in high-grade glioma during and after postoperative radiotherapy. Anticancer Res 31:3559–3565
Cha J, Kim ST, Kim HJ et al (2014) Differentiation of tumor progression from pseudoprogression in patients with posttreatment glioblastoma using multiparametric histogram analysis. AJNR Am J Neuroradiol 35:1309–1317
Park JE, Kim HS, Goh MJ et al (2015) Pseudoprogression in patients with glioblastoma: assessment by using volume-weighted voxel-based multiparametric clustering of MR imaging data in an independent test set. Radiology 275:792–802
Caroline I, Rosenthal MA (2012) Imaging modalities in high-grade gliomas: pseudoprogression, recurrence, or necrosis? J Clin Neurosci 19:633–637
Terakawa Y, Tsuyuguchi N, Iwai Y et al (2008) Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 49:694–699
Galldiks N, Dunkl V, Stoffels GJ et al (2015) Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur J Nucl Med Mol Imaging 42:685–695
Batchelor TT, Sorensen AG, di Tomaso E et al (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11:83–95
Melhem ER, Mehta NR (1999) Dynamic T1-weighted spin-echo MR imaging: the role of digital subtraction in the demonstration of enhancing brain lesions. J Magn Reson Imaging 9:503–508
Kanaly CW, Ding D, Mehta AI et al (2011) A novel method for volumetric MRI response assessment of enhancing brain tumors. PLoS One 6:e16031
Ellingson BM, Kim HJ, Woodworth DC et al (2014) Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology 271:200–210
Sorensen AG, Batchelor TT, Zhang WT et al (2009) A "vascular normalization index" as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res 69:5296–5300
Galldiks N, Law I, Pope WB et al (2017) The use of amino acid PET and conventional MRI for monitoring of brain tumor therapy. Neuroimage Clin 13:386–394
Galldiks N, Filss CP, Goldbrunner R, Langen KJ (2012) Discrepant MR and [(18)F]fluoroethyl-L-tyrosine PET imaging findings in a patient with bevacizumab failure. Case Rep Oncol 5:490–494
Galldiks N, Rapp M, Stoffels G et al (2013) Earlier diagnosis of progressive disease during bevacizumab treatment using O-(2-18F-fluorethyl)-L-tyrosine positron emission tomography in comparison with magnetic resonance imaging. Mol Imaging 12:273–276
Aquino D, Gioppo A, Finocchiaro G et al (2017) MRI in glioma immunotherapy: evidence, pitfalls, and perspectives. J Immunol Res 2017:5813951
Vrabec M, Van Cauter S, Himmelreich U et al (2011) MR perfusion and diffusion imaging in the follow-up of recurrent glioblastoma treated with dendritic cell immunotherapy: a pilot study. Neuroradiology 53:721–731
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Tamrazi, B., Mankad, K., Nelson, M. et al. Current concepts and challenges in the radiologic assessment of brain tumors in children: part 2. Pediatr Radiol 48, 1844–1860 (2018). https://doi.org/10.1007/s00247-018-4232-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-018-4232-7