Abstract
Purpose
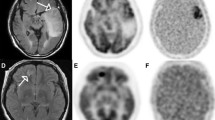
Glioblastoma multiforme (GBM) is characterized by tissue hypoxia associated with resistance to radiotherapy and chemotherapy. To clarify the biological link between hypoxia and tumour-induced neovascularization and tumour aggressiveness, we analysed detailed volumetric and spatial information of viable hypoxic tissue assessed by 18F-fluoromisonidazole (FMISO) PET relative to neovascularization in Gd-enhanced MRI and tumour aggressiveness by L-methyl-11C-methionine (MET) PET in newly diagnosed GBMs.
Methods
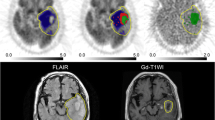
Ten patients with newly diagnosed GBMs were investigated with FMISO PET, MET PET and Gd-enhanced MRI before surgery. Tumour volumes were calculated by performing a three-dimensional threshold-based volume of interest (VOI) analysis for metabolically active volume on MET PET (MET uptake indices of ≥1.3 and ≥1.5) and Gd-enhanced volume on MRI. FMISO PET was scaled to the blood FMISO activity to create tumour to blood (T/B) images. The hypoxic volume (HV) was defined as the region with T/B greater than 1.2. PET and MR images of each patient were coregistered to analyse the spatial location of viable hypoxic tissue relative to neovascularization and active tumour extension.
Results
Metabolically active tumour volumes defined using MET uptake indices of ≥1.3 and ≥1.5 and the volumes of Gd enhancement showed a strong correlation (r = 0.86, p < 0.01 for an index of ≥1.3 and r = 0.77, p < 0.05 for an index of ≥1.5). The HVs were also excellently correlated with the volumes of Gd enhancement (r = 0.94, p < 0.01). The metabolically active tumour volumes as defined by a MET uptake index of ≥1.3 and the HVs exhibited a strong correlation (r = 0.87, p < 0.01). On superimposed images, the metabolically active area on MET PET defined by a MET uptake index of ≥1.3 was usually larger than the area of the Gd enhancement and about 20–30% of the MET area extended outside the area of the enhancement. On the other hand, the surface area of viable hypoxic tissue with a T/B cutoff of ≥1.2 on FMISO PET did not substantially differ from the area of the Gd enhancement.
Conclusion
The volumetric analysis demonstrates that the viable hypoxic tissue assessed by FMISO PET is related to the neovascularization in Gd-enhanced MRI and the tumour aggressiveness by MET PET in newly diagnosed GBMs. The spatial analysis shows that the metabolically active tumour may be substantially underestimated by Gd-enhanced MRI. Complementary use of MET and FMISO to Gd-enhanced MRI may improve the understanding of tumour biology and lead to the most efficient delineation of tumour volume and treatment strategy.





Similar content being viewed by others
References
Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol 2007;25:4127–36.
Swanson KR, Alvord Jr EC, Murray JD. Virtual brain tumours (gliomas) enhance the reality of medical imaging and highlight inadequacies of current therapy. Br J Cancer 2002;86:14–8.
Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg 1987;66:865–74.
Rajendran JG, Mankoff DA, O’Sullivan F, Peterson LM, Schwartz DL, Conrad EU, et al. Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res 2004;10:2245–52.
Brat DJ, Castellano-Sanchez AA, Hunter SB, Pecot M, Cohen C, Hammond EH, et al. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res 2004;64:920–7.
Fischer I, Gagner JP, Law M, Newcomb EW, Zagzag D. Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol 2005;15:297–310.
Tsang RW, Fyles AW, Milosevic M, Syed A, Pintille M, Levin W, et al. Interrelationship of proliferation and hypoxia in carcinoma of the cervix. Int J Radiat Oncol Biol Phys 2000;46:95–9.
Slomiany MG, Rosenzweig SA. Hypoxia-inducible factor-1-dependent and -independent regulation of insulin-like growth factor-1-stimulated vascular endothelial growth factor secretion. J Pharmacol Exp Ther 2006;318:666–75.
Burger PC, Vogel FS, Green SB, Strike TA. Glioblastoma multiforme and anaplastic astrocytoma. Pathologic criteria and prognostic implications. Cancer 1985;56:1106–11.
Daumas-Duport C, Scheithauer B, O’Fallon J, Kelly P. Grading of astrocytomas. A simple and reproducible method. Cancer 1988;62:2152–65.
Rasey JS, Koh WJ, Evans ML, Peterson LM, Lewellen TK, Graham MM, et al. Quantifying regional hypoxia in human tumors with positron emission tomography of [18F]fluoromisonidazole: a pretherapy study of 37 patients. Int J Radiat Oncol Biol Phys 1996;36:417–26.
Valk PE, Mathis CA, Prados MD, Gilbert JC, Budinger TF. Hypoxia in human gliomas: demonstration by PET with fluorine-18-fluoromisonidazole. J Nucl Med 1992;33:2133–7.
Swanson KR, Chakraborty G, Wang CH, Rockne R, Harpold HLP, Muzi M, et al. Complementary but distinct roles for MRI and 18F-fluoromisonidazole PET in the assessment of human glioblastomas. J Nucl Med 2009;50:36–44.
Herholz K, Hölzer T, Bauer B, Schröder R, Voges J, Ernestus R-I, et al. 11C-methionine PET for differential diagnosis of low-grade gliomas. Neurology 1998;50:1316–22.
Kracht LW, Miletic H, Busch S, Jacobs AH, Voges J, Hoevels M, et al. Delineation of brain tumor extent with [11C]L-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res 2004;10:7163–70.
Ceyssens S, Van Laere K, de Groot T, Goffin J, Bormans G, Mortelmans L. [11C]methionine PET, histopathology, and survival in primary brain tumors and recurrence. AJNR Am J Neuroradiol 2006;27:1432–7.
Hatakeyama T, Kawai N, Nishiyama Y, Yamamoto Y, Sasakawa Y, Ichikawa T, et al. 11C-methionine (MET) and 18F-fluorothymidine (FLT) PET in patients with newly diagnosed glioma. Eur J Nucl Med Mol Imaging 2008;35:2009–17.
Nariai T, Tanaka Y, Wakimoto H, Aoyagi M, Tamaki M, Ishikawa K, et al. Usefulness of L-[methyl-11C] methionine-positron emission tomography as a biological monitoring tool in the treatment of glioma. J Neurosurg 2005;103:498–507.
Kim S, Chung J-K, Im S-H, Jeong JM, Lee DS, Kim DG, et al. 11C-methionine PET as a prognostic marker in patients with glioma: comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging 2005;32:52–9.
Mosskin M, Ericson K, Hindmarsh T, von Holst H, Collins VP, Bergström M, et al. Positron emission tomography compared with magnetic resonance imaging and computed tomography in supratentorial gliomas using multiple stereotactic biopsies as reference. Acta Radiol 1989;30:225–32.
Miwa K, Shinoda J, Yano H, Okumura A, Iwama T, Nakashima T, et al. Discrepancy between lesion distributions on methionine PET and MR images in patients with glioblastoma multiforme: insight from a PET and MR fusion image study. J Neurol Neurosurg Psychiatry 2004;75:1457–62.
Pirotte B, Goldman S, Dewitte O, Massager N, Wikler D, Lefranc F, et al. Integrated positron emission tomography and magnetic resonance imaging-guided resection of brain tumors: a report of 103 consecutive procedures. J Neurosurg 2006;104:238–53.
Ishiwata K, Ido T, Vaalburg W. Increased amounts of D-enantiomer dependent on alkaline concentration in the synthesis of L-[methyl-11C]methionine. Int J Rad Appl Instrum A 1988;39:311–4.
Lim JL, Berridge MS. An efficient radiosynthesis of [18F]fluoromisonidasole. Appl Radiat Isot 1993;44:1085–91.
Galldiks N, Ullrich R, Schroeter M, Fink GR, Jacobs AH, Kracht LW. Volumetry of [(11)C]-methionine PET uptake and MRI contrast enhancement in patients with recurrent glioblastoma multiforme. Eur J Nucl Med Mol Imaging 2010;37:84–92.
Kinoshita M, Hashimoto N, Goto T, Yanagisawa T, Okita Y, Kagawa N, et al. Use of fractional anisotropy for determination of the cut-off value in 11C-methionine positron emission tomography for glioma. Neuroimage 2009;45:312–8.
Spence AM, Muzi M, Swanson KR, O’Sullivan F, Rockhill JK, Rajendran JG, et al. Regional hypoxia in glioblastoma multiforme quantified with [18F]fluoromisonidazole positron emission tomography before radiotherapy: correlation with time to progression and survival. Clin Cancer Res 2008;14:2623–30.
Szeto MD, Chakraborty G, Handley J, Rockne R, Muzi M, Alvord Jr EC, et al. Quantitative metrics of net proliferation and invasion link biological aggressiveness assessed by MRI with hypoxia assessed by FMISO-PET in newly diagnosed glioblastomas. Cancer Res 2009;69:4502–9.
Shimada Y, Uemura K, Ardekani BA, Nagaoka T, Ishiwata K, Toyama H, et al. Application of PET-MRI registration techniques to cat brain imaging. J Neurosci Methods 2000;101:1–7.
Rampling R, Cruickshank G, Lewis AD, Fitzsimmons SA, Workman P. Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. Int J Radiat Oncol Biol Phys 1994;29:427–31.
Evans SM, Judy KD, Dunphy I, Jenkins WT, Nelson PT, Collins R, et al. Comparative measurements of hypoxia in human brain tumors using needle electrodes and EF5 binding. Cancer Res 2004;64:1886–92.
Rasey JS, Nelson NJ, Chin L, Evans ML, Grunbaum Z. Characteristics of the binding of labeled fluoromisonidazole in cells in vitro. Radiat Res 1990;122:301–8.
Cher LM, Murone C, Lawrentschuk N, Ramdave S, Papenfuss A, Hannah A, et al. Correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in gliomas using 18F-fluoromisonidazole, 18F-FDG PET, and immunohistochemical studies. J Nucl Med 2006;47:410–8.
Evans SM, Judy KD, Dunphy I, Jenkins WT, Hwang WT, Nelson PT, et al. Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res 2004;10:8177–84.
Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ 2008;15:678–85.
Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL. Expression of hypoxia-inducible factor 1α in brain tumors: association with angiogenesis, invasion, and progression. Cancer 2000;88:2606–18.
Kleinberg L, Grossman SA, Carson K, Lesser G, O’Neill A, Pearlman J, et al. Survival of patients with newly diagnosed glioblastoma multiforme treated with RSR13 and radiotherapy: results of a phase II new approaches to brain tumor therapy CNS consortium safety and efficacy study. J Clin Oncol 2002;20:3149–55.
Del Rowe J, Scott C, Werner-Wasik M, Bahary JP, Curran WJ, Urtasun RC, et al. Single-arm, open-label phase II study of intravenously administered tirapazamine and radiation therapy for glioblastoma multiforme. J Clin Oncol 2000;18:1254–9.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawai, N., Maeda, Y., Kudomi, N. et al. Correlation of biological aggressiveness assessed by 11C-methionine PET and hypoxic burden assessed by 18F-fluoromisonidazole PET in newly diagnosed glioblastoma. Eur J Nucl Med Mol Imaging 38, 441–450 (2011). https://doi.org/10.1007/s00259-010-1645-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-010-1645-4