Abstract
Purpose
The aim of this study was to investigate the relationship between 123I-metaiodobenzylguanidine (MIBG) scan semi-quantification and a new 18F-DOPA positron emission tomography (PET)/CT score in patients with suspected or documented neuroblastoma (NB) relapse and to assess the association between these two parameters and progression-free survival (PFS)/overall survival (OS).
Methods
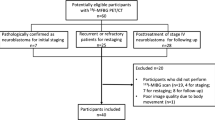
We analysed 24 NB patients who had undergone 123I-MIBG and 18F-DOPA PET/CT scans at the time of suspected relapse, after applying a proper scoring system for each scan. In time-to-event analyses, the score distributions were regarded as continuous and were categorized in tertiles and medians. We used Kaplan-Meier curves and Cox proportional hazard models for PFS and OS in order to estimate the independent prognostic impact of 123I-MIBG and 18F-DOPA PET/CT scans.
Results
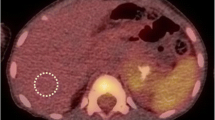
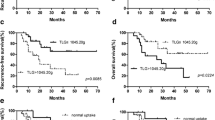
The 123I-MIBG and 18F-DOPA scores were highly and positively correlated (Spearman’s rho = 0.8, p < 0.001). Over a median follow-up of 14 months (range 6–82), 12 cases of disease progression and 6 deaths occurred. Multivariate Cox models showed a higher risk of disease progression [hazard ratio (HR) 17.0, 95 % confidence interval (CI) 2.7–109] in NB patients with 123I-MIBG score > 3 (3rd tertile) and an even higher risk (HR:37.2, 95 % CI 2.4–574) in those with 18F-DOPA whole-body metabolic burden (WBMB) >7.5 (median), after adjustment for all main clinical/pathological factors considered. Kaplan-Meier analyses showed a significant association with OS (log-rank p = 0.01 and p = 0.03 for 123I-MIBG and 18F-DOPA WBMB, respectively).
Conclusion
Our results confirm the good agreement between 18F-DOPA PET/CT and 123I-MIBG scan in patients affected by NB relapse. In time-to-event analyses, 123I-MIBG scan and 18F-DOPA PET/CT scores were independently and significantly associated with disease progression.






Similar content being viewed by others
References
Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med 1999;341:1165–73.
Pearson AD, Pinkerton CR, Lewis IJ, Imeson J, Ellershaw C, Machin D, et al. High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: a randomised trial. Lancet Oncol 2008;9:247–56.
Zage PE, Kletzel M, Murray K, Marcus R, Castleberry R, Zhang Y, et al. Outcomes of the POG 9340/9341/9342 trials for children with high-risk neuroblastoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer 2008;51:747–53.
London WB, Castel V, Monclair T, Ambros PF, Pearson AD, Cohn SL, et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: a report from the International Neuroblastoma Risk Group project. J Clin Oncol 2011;29:3286–92.
Jacobson AF, Deng H, Lombard J, Lessig HJ, Black RR.123I-meta-iodobenzylguanidine scintigraphy for the detection of neuroblastoma and pheochromocytoma: results of a meta-analysis. J Clin Endocrinol Metab 2010;95:2596–606.
Lewington V, Bar Sever Z, Giammarile F, Lynch T, McEwan A, Shulkin B, et al. Development of a semi-quantitative I-123 mIBG reporting method in high risk neuroblastoma. J Nucl Med 2009;50:1379.
Matthay KK, Edeline V, Lumbroso J, Tanguy ML, Asselain B, Zucker JM, et al. Correlation of early metastatic response by123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J Clin Oncol 2003;21:2486–91.
Papathanasiou ND, Gaze MN, Sullivan K, Aldridge M, Waddington W, Almuhaideb A, et al. 18F-FDG PET/CT and 123I-metaiodobenzylguanidine imaging in high-risk neuroblastoma: diagnostic comparison and survival analysis. J Nucl Med 2011;52:519–25.
Messina JA, Cheng SC, Franc BL, Charron M, Shulkin B, To B, et al. Evaluation of semi-quantitative scoring system for metaiodobenzylguanidine (mIBG) scans in patients with relapsed neuroblastoma. Pediatr Blood Cancer 2006;47:865–74.
Piccardo A, Lopci E, Conte M, Garaventa A, Foppiani L, Altrinetti V, et al. Comparison of (18)F-dopa PET/CT and (123)I-MIBG scintigraphy in stage 3 and 4 neuroblastoma: a pilot study. Eur J Nucl Med Mol Imaging 2012;39:57–61.
Lopci E, Piccardo A, Nanni C, Altrinetti V, Garaventa A, Pession A, et al. 18F-DOPA PET/CT in neuroblastoma: comparison of conventional imaging with CT/MR. Clin Nucl Med 2012;37:e73–8.
Lu MY, Liu YL, Chang HH, Jou ST, Yang YL, Lin KH, et al. Characterization of neuroblastic tumors using 18F-FDOPA PET. J Nucl Med 2013;54:42–9.
ICRP. Radiation dose to patients from radiopharmaceuticals. Addendum 3 to ICRP publication 53. ICRP publication 106. Ann ICRP 2008;38(1–2):1–197.
ICRP. Radiation dose to patients from radiopharmaceuticals (addendum 2 to ICRP publication 53). ICRP Publication 80. Ann ICRP 1998;28(3):1–126.
Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 1993;11:1466–77.
Lassmann M, Biassoni L, Monsieurs M, Franzius C, Jacobs F, EANM Dosimetry and Paediatrics Committees. The new EANM paediatric dosage card. Eur J Nucl Med Mol Imaging 2007;34:796–8.
Matthay KK, Shulkin B, Ladenstein R, Michon J, Giammarile F, Levington V, et al. Criteria for evaluation of disease extent by 123I-metaiodobenzylguanidine scans in neuroblastoma: a report for the International Neuroblastoma Risk Group (INRG) Task Force. Br J Cancer 2010;102:1319–22.
Olivier P, Colarinha P, Fettich J, Fischer S, Frökier J, Giammarile F, et al. Guidelines for radioiodinated MIBG scintigraphy in children. Eur J Nucl Med Mol Imaging 2003;30:B45–50.
Luxen A, Perlmutter M, Bida GT, Van Moffaert G, Cook JS, Satyamurthy N, et al. Remote, semiautomated production of 6-[18F]fluoro-L-dopa for human studies with PET. Int J Rad Appl Instrum A 1990;41:275–81.
Fiebrich HB, Brouwers AH, Kerstens MN, Pijl ME, Kema IP, de Jong JR, et al. 6-[F-18]Fluoro-L-dihydroxyphenylalanine positron emission tomography is superior to conventional imaging with (123)I-metaiodobenzylguanidine scintigraphy, computer tomography, and magnetic resonance imaging in localizing tumors causing catecholamine excess. J Clin Endocrinol Metab 2009;94:3922–30.
Berkowitz A, Basu S, Srinivas S, Sankaran S, Schuster S, Alavi A. Determination of whole-body metabolic burden as a quantitative measure of disease activity in lymphoma: a novel approach with fluorodeoxyglucose-PET. Nucl Med Commun 2008;29:521–6.
Okuyama C, Ushijima Y, Kubota T, Nakamura T, Kikkawa M, Nishimura T. Utility of follow-up studies using meta-[123 I]iodobenzylguanidine scintigraphy for detecting recurrent neuroblastoma. Nucl Med Commun 2002;23:663–72.
Kushner BH, Kramer K, Modak S, Cheung NK. Sensitivity of surveillance studies for detecting asymptomatic and unsuspected relapse of high-risk neuroblastoma. J Clin Oncol 2009;27:1041–6.
Conte M, Parodi S, De Bernardi B, Milanaccio C, Mazzocco K, Angelini P, et al. Neuroblastoma in adolescents: the Italian experience. Cancer 2006;106:1409–17.
Morgenstern DA, Baruchel S, Irwin MS. Current and future strategies for relapsed neuroblastoma: challenges on the road to precision therapy. J Pediatr Hematol Oncol 2013;35:337–47.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Stefano Fanti and Alberto Garaventa share senior co-authorship.
Rights and permissions
About this article
Cite this article
Piccardo, A., Puntoni, M., Lopci, E. et al. Prognostic value of 18F-DOPA PET/CT at the time of recurrence in patients affected by neuroblastoma. Eur J Nucl Med Mol Imaging 41, 1046–1056 (2014). https://doi.org/10.1007/s00259-014-2691-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2691-0