Abstract
Objectives
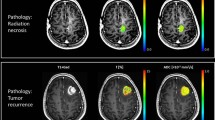
To evaluate the added value of diffusion-weighted imaging (DWI) to perfusion-weighted imaging (PWI) for differentiating tumour progression from radiation necrosis.
Methods
Sixteen consecutive patients who underwent removal of a metastatic brain tumour that increased in size after stereotactic radiosurgery were retrospectively reviewed. The layering of the ADC was categorised into three patterns. ADC values were measured on each layer, and the maximum rCBV was measured. rCBV and the layering pattern of the ADC of radiation necrosis and tumour progression were compared.
Results
Nine cases of radiation necrosis and seven cases of tumour progression were pathologically confirmed. Radiation necrosis (88.9 % vs. 14.3 %) showed a three-layer pattern of ADC with a middle layer of minimum ADC more frequently. If rCBV larger than 2.6 was used to differentiate radiation necrosis and tumour progression, the sensitivity was 100 % but specificity was 56 %. If the lesions with the three-layer pattern of ADC with moderately increased rCBV (2.6–4.1) were excluded from tumour progression, the sensitivity and specificity increased to 100 %.
Conclusions
The three-layer pattern of ADC shows high specificity in diagnosing radiation necrosis; therefore, combined analysis of the ADC pattern with rCBV may have added value in the correct differentiation of tumour progression from radiation necrosis.
Key Points
•MRI follow-up often reveals increasing size of enhancing lesions after stereotactic radiosurgery
•Inflammation around tumour necrosis increases regional cerebral blood volume (rCBV), mimicking progression
•A three-layer apparent diffusion coefficient (ADC) pattern on diffusion-weighted MRI suggests radiation necrosis.
•The combined use of rCBV and DW MRI enables accurate differentiation.






Similar content being viewed by others
References
Huber PE, Hawighorst H, Fuss M, van Kaick G, Wannenmacher MF, Debus J (2001) Transient enlargement of contrast uptake on MRI after linear accelerator (LINAC) stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys 49:1339–1349
Da Silva AN, Nagayama K, Schlesinger D, Sheehan JP (2009) Early brain tumor metastasis reduction following Gamma Knife surgery. J Neurosurg 110:547–552
Patel TR, McHugh BJ, Bi WL, Minja FJ, Knisely JP, Chiang VL (2011) A comprehensive review of MR imaging changes following radiosurgery to 500 brain metastases. AJNR Am J Neuroradiol 32:1885–1892
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Vannucci L (2010) To suppress to rescue? Changing the approach for recalling anticancer immune responses. Front Biosci (Schol Ed) 2:1189–1197
Hamai A, Benlalam H, Meslin F et al (2010) Immune surveillance of human cancer: if the cytotoxic T-lymphocytes play the music, does the tumoral system call the tune? Tissue Antigens 75:1–8
Rabin BM, Meyer JR, Berlin JW, Marymount MH, Palka PS, Russell EJ (1996) Radiation-induced changes in the central nervous system and head and neck. Radiographics 16:1055–1072
Gourmelon P, Marquette C, Agay D, Mathieu J, Clarencon D (2005) Involvement of the central nervous system in radiation-induced multi-organ dysfunction and/or failure. BJR Suppl/BIR 27:62–68
Chiang CS, Hong JH, Stalder A, Sun JR, Withers HR, McBride WH (1997) Delayed molecular responses to brain irradiation. Int J Radiat Biol 72:45–53
Yoshii Y (2008) Pathological review of late cerebral radionecrosis. Brain Tumor Pathol 25:51–58
Asao C, Korogi Y, Kitajima M et al (2005) Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am J Neuroradiol 26:1455–1460
Tung GA, Evangelista P, Rogg JM, Duncan JA 3rd (2001) Diffusion-weighted MR imaging of rim-enhancing brain masses: is markedly decreased water diffusion specific for brain abscess? AJR. Am J Roentgenol 177:709–712
Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR (1996) High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med: Off J Soc Magn Reson Med/Soc Magn Reson Med 36:715–725
Boxerman JL, Schmainda KM, Weisskoff RM (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 27:859–867
Pan HC, Sheehan J, Stroila M, Steiner M, Steiner L (2005) Gamma knife surgery for brain metastases from lung cancer. J Neurosurg 102:128–133
Desprechins B, Stadnik T, Koerts G, Shabana W, Breucq C, Osteaux M (1999) Use of diffusion-weighted MR imaging in differential diagnosis between intracerebral necrotic tumors and cerebral abscesses. AJNR Am J Neuroradiol 20:1252–1257
Jagannathan J, Bourne TD, Schlesinger D et al (2010) Clinical and pathological characteristics of brain metastasis resected after failed radiosurgery. Neurosurgery 66:208–217
Oh BC, Pagnini PG, Wang MY et al (2007) Stereotactic radiosurgery: adjacent tissue injury and response after high-dose single fraction radiation: Part I—Histology, imaging, and molecular events. Neurosurgery 60:31–44, discussion 44–35
Monabati A, Kumar PV, Kamkarpour A (2000) Intraoperative cytodiagnosis of metastatic brain tumors confused clinically with brain abscess. A report of three cases. Acta Cytol 44:437–441
Holtas S, Geijer B, Stromblad LG, Maly-Sundgren P, Burtscher IM (2000) A ring-enhancing metastasis with central high signal on diffusion-weighted imaging and low apparent diffusion coefficients. Neuroradiology 42:824–827
Biousse V, Newman NJ, Hunter SB, Hudgins PA (2003) Diffusion weighted imaging in radiation necrosis. J Neurol Neurosurg Psychiatry 74:382–384
Kang TW, Kim ST, Byun HS et al (2009) Morphological and functional MRI, MRS, perfusion and diffusion changes after radiosurgery of brain metastasis. Eur J Radiol 72:370–380
Toh CH, Wei KC, Ng SH, Wan YL, Lin CP, Castillo M (2011) Differentiation of brain abscesses from necrotic glioblastomas and cystic metastatic brain tumors with diffusion tensor imaging. AJNR Am J Neuroradiol 32:1646–1651
Cha S (2006) Update on brain tumor imaging: from anatomy to physiology. AJNR Am J Neuroradiol 27:475–487
Wong JC, Provenzale JM, Petrella JR (2000) Perfusion MR imaging of brain neoplasms. AJR Am J Roentgenol 174:1147–1157
Essig M, Waschkies M, Wenz F, Debus J, Hentrich HR, Knopp MV (2003) Assessment of brain metastases with dynamic susceptibility-weighted contrast-enhanced MR imaging: initial results. Radiology 228:193–199
Mitsuya K, Nakasu Y, Horiguchi S et al (2010) Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. J Neurooncol 99:81–88
Jain R, Narang J, Schultz L et al (2011) Permeability estimates in histopathology-proved treatment-induced necrosis using perfusion CT: can these add to other perfusion parameters in differentiating from recurrent/progressive tumors? AJNR Am J Neuroradiol 32:658–663
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cha, J., Kim, S.T., Kim, HJ. et al. Analysis of the layering pattern of the apparent diffusion coefficient (ADC) for differentiation of radiation necrosis from tumour progression. Eur Radiol 23, 879–886 (2013). https://doi.org/10.1007/s00330-012-2638-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-012-2638-4