Abstract
Purpose
Late gadolinium enhancement cardiac MR (LGE-CMR) and extracellular volume fraction (ECV-CMR) are widely used to evaluate macroscopic and microscopic myocardial fibrosis. Macrocyclic contrast media are increasingly used off-label for myocardial scar assessment, given the superior safety profile of these agents. We aimed to assess the performance of two macrocyclic contrast agents, gadoterate meglumine and gadobutrol, for the evaluation of myocardial scar.
Material and methods
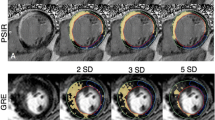
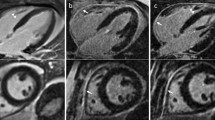
Forty subjects (61 ± 11 years, 67.5% men) who underwent LGE-CMR using gadobutrol were prospectively recruited for a research CMR scan using same-dose gadoterate meglumine (0.2 mmol/kg) at 1.5 T. Myocardial scar quantification was performed using a short-axis phase-sensitive inversion recovery (PSIR) Turbo-FLASH and steady-state free precession (SSFP) images. Pre- and post-contrast T1-mapping was employed to assess myocardial ECV. An intraclass correlation coefficient (ICC) was used to check for reliability between the two contrast agents.
Results
Using manual thresholding on PSIR Turbo-FLASH images, mean LGE scar percentage (LGE%) was 9.9 ± 9.7% and 9.4 ± 9.7% for gadobutrol and gadoterate meglumine, respectively (p > 0.05) (ICC: 0.99, 95% CI: 0.97–0.99). Using the PSIR SSFP technique and manual thresholding, LGE% averaged 7.5 ± 9.0% and 7.1 ± 8.6% for gadobutrol and gadoterate meglumine, respectively (p > 0.05) (ICC: 0.99, 95% CI: 0.98–0.99). Average ECV with gadobutrol and gadoterate meglumine were similar at 28.40 ± 4.88 and 28.46 ± 4.73 (p > 0.05) with a strong correlation (ICC: 0.98, 95% CI: 0.94–0.99).
Conclusion
We found LGE- and ECV-CMR values derived from gadoterate meglumine comparable to values derived from gadobutrol. Gadoterate meglumine has a comparable performance to gadobutrol in identifying LGE-derived myocardial scar both qualitatively and quantitatively.
Key Points
• Late gadolinium-enhancement cardiac MR (LGE-MR) and extracellular volume (ECV) fraction are widely used to evaluate macroscopic and microscopic myocardial fibrosis.
• Macrocyclic contrast media are increasingly used off-label for myocardial scar assessment, given the presumed superior safety profile of these agents.
• LGE- and ECV-CMR values derived from gadoterate meglumine are comparable to values derived from gadobutrol.





Similar content being viewed by others
Abbreviations
- CI:
-
Confidence interval
- CMR:
-
Cardiac magnetic resonance
- CNR:
-
Contrast-to-noise ratio
- ECV:
-
Extracellular volume
- GBCA:
-
Gadolinium-based contrast agents
- GFR:
-
Glomerular filtration rate
- ICC:
-
Intraclass correlation coefficients
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricle
- MOLLI:
-
Modified Look-Locker inversion recovery
- PSIR:
-
Phase-sensitive inversion recovery
- SI:
-
Signal intensity
- SNR:
-
Signal-to-noise ratio
- SSFP:
-
Steady-state free precession
References
Kino A, Zuehlsdorff S, Sheehan JJ et al (2009) Three-dimensional phase-sensitive inversion-recovery turbo FLASH sequence for the evaluation of left ventricular myocardial scar. AJR Am J Roentgenol 193(5):W381–W388
Kim RJ, Fieno DS, Parrish TB et al (1999) Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 100(19):1992–2002
Schelbert EB, Hsu LY, Anderson SA et al (2010) Late gadolinium-enhancement cardiac magnetic resonance identifies postinfarction myocardial fibrosis and the border zone at the near cellular level in ex vivo rat heart. Circ Cardiovasc Imaging 3(6):743–752
Gulati A, Jabbour A, Ismail TF et al (2013) Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 309(9):896–908
Kim RJ, Wu E, Rafael A et al (2000) The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 343(20):1445–1453
Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA (2011) Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol 57(8):891–903
Ambale-Venkatesh B, Lima JA (2015) Cardiac MRI: a central prognostic tool in myocardial fibrosis. Nat Rev Cardiol 12(1):18–29
Kellman P, Wilson JR, Xue H, Ugander M, Arai AE (2012) Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson 14:63
Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S (2016) Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson 18(1):89
Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC (2014) Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol 49(10):685–690
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270(3):834–841
Kanda T, Osawa M, Oba H et al (2015) High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology 275(3):803–809
Idée JM, Port M, Robic C, Medina C, Sabatou M, Corot C (2009) Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J Magn Reson Imaging 30(6):1249–1258
Kim RJ, Shah DJ, Judd RM (2003) How we perform delayed enhancement imaging. J Cardiovasc Magn Reson 5(3):505–514
Messroghli DR, Greiser A, Fröhlich M, Dietz R, Schulz-Menger J (2007) Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging 26(4):1081–1086
Stirrat J, Joncas SX, Salerno M, Drangova M, White J (2015) Influence of phase correction of late gadolinium enhancement images on scar signal quantification in patients with ischemic and non-ischemic cardiomyopathy. J Cardiovasc Magn Reson 17:66
Vermes E, Childs H, Carbone I, Barckow P, Friedrich MG (2013) Auto-threshold quantification of late gadolinium enhancement in patients with acute heart disease. J Magn Reson Imaging 37(2):382–390
Mikami Y, Kolman L, Joncas SX et al (2014) Accuracy and reproducibility of semi-automated late gadolinium enhancement quantification techniques in patients with hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 16:85
Dietrich O, Raya JG, Reeder SB, Reiser MF, Schoenberg SO (2007) Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging 26(2):375–385
Cerqueira MD, Weissman NJ, Dilsizian V et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105(4):539–542
Moon JC, Messroghli DR, Kellman P et al (2013) Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of cardiology consensus statement. J Cardiovasc Magn Reson 15:92
Assomull RG, Prasad SK, Lyne J et al (2006) Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol 48(10):1977–1985
Bello D, Fieno DS, Kim RJ et al (2005) Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol 45(7):1104–1108
Wagner A, Mahrholdt H, Holly TA et al (2003) Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet 361(9355):374–379
Wagner M, Schilling R, Doeblin P et al (2013) Macrocyclic contrast agents for magnetic resonance imaging of chronic myocardial infarction: intraindividual comparison of gadobutrol and gadoterate meglumine. Eur Radiol 23(1):108–114
Huber A, Schoenberg SO, Spannagl B et al (2006) Single-shot inversion recovery TrueFISP for assessment of myocardial infarction. AJR Am J Roentgenol 186(3):627–633
Gai N, Turkbey EB, Nazarian S et al (2011) T1 mapping of the gadolinium-enhanced myocardium: adjustment for factors affecting interpatient comparison. Magn Reson Med 65(5):1407–1415
Kawel N, Nacif M, Zavodni A et al (2012) T1 mapping of the myocardium: intra-individual assessment of post-contrast T1 time evolution and extracellular volume fraction at 3T for Gd-DTPA and Gd-BOPTA. J Cardiovasc Magn Reson 14:26
Acknowledgements
We would like to particularly thank all the patients who participated in our study.
Funding
This study has received funding by Geurbet, LLC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Professor James C. Carr, MD.
Conflict of interest
Dr. James Carr and Dr. Jeremy Collins are members of the advisory board of Guerbet, LLC.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Methodology
• Prospective
• Diagnostic study
• Performed at one institution
Rights and permissions
About this article
Cite this article
Rahsepar, A.A., Ghasemiesfe, A., Suwa, K. et al. Comprehensive evaluation of macroscopic and microscopic myocardial fibrosis by cardiac MR: intra-individual comparison of gadobutrol versus gadoterate meglumine. Eur Radiol 29, 4357–4367 (2019). https://doi.org/10.1007/s00330-018-5956-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5956-3