Abstract
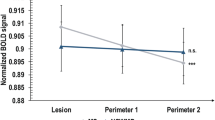
Multiple sclerosis (MS) plaques and age related white matter lesions (WML) are of similar morphological appearance on T2 weighted MRI. Therefore their differentiation is sometimes crucial. Proton magnetic resonance spectroscopy (1H–MRS) adds metabolic information to conventional imaging and may help to distinguish inflammatory MS plaques from vascular related WML. This study was performed to evaluate the metabolite pattern in MS plaques and WML. 15 MS patients, 14 elderly individuals with WML and 16 controls were investigated by conventional MRI and short echo quantitative 1H–MRS (TE: 30ms, TR: 3000ms). The mean metabolite concentrations in normal control white matter (NCWM), MS plaques and WML were: t–NAA:
8.96 mmol/l (SD:0.93) vs
6.79 mmol/l (SD: 1.99) vs
7.18 mmol/l (SD: 1.41); Cho:
1.66 mmol/l (SD: 0.4) vs
1.49 mmol/l (SD: 0.45) vs
1.46 mmol/l (SD: 0.34); PCr:
5.64 mmol/l (SD: 0.83) vs
4.9mmol/l (SD: 1.3) vs 4.95 mmol/l (SD: 0.86); myo–Ins: 4.57 mmol/l (SD:1.05) vs 6.34 mmol/l (SD: 2.03) vs 4.5 mmol/l (SD: 0.96). t–NAA reduction in MS plaques and WML was significant compared with controls (p ≤ 0.001). MS plaques showed significantly elevated myo– Ins concentrations compared with controls (p = 0.002) and to WML (p = 0.003). In summary MS plaques and WML show a decrease in their t–NAA concentrations compared with controls. Elevated concentrations of myo–Ins in MS plaques but not in WML makes this metabolite of special interest for their differentiation.
Similar content being viewed by others

References
Lassmann H (1998) Neuropathology in multiple sclerosis: new concepts. Mult Scler 4:93–98
Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H (1993) Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 43:1683–1689
Fazekas F, Offenbacher H, Fuchs S, Schmidt R, Niederkorn K, Horner S, Lechner H (1988) Criteria for an increased specificity of MRI interpretation in elderly subjects with suspected multiple sclerosis. Neurology 38:1822–1825
Barkhof F, Filippi M, Miller D, Scheltens P, Campi A, Polman C, Comi G, Ader H, Losseff N, Valk J (1997) Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 120:2059–2069
Paty DW, Kastrukoff LF, Hashimoto SA, Hooge JP, Eisen AA, Eisen KA, Purves SJ, Low MD, Brandejs V (1988) MRI in the diagnosis of MS: a prospective study with comparison of clinical evaluation, evoked potentials, oligoclonal banding, and CT. Neurology 38:180–185
Offenbacher H, Fazekas F, Schmidt R, Freidl W, Flooh E, Payer F, Lechner A (1993) Assessment of MRI criteria for a diagnosis of MS. Neurology 43:905–909
Bitsch A, Bruhn H, Vougioukas V, Stringaris A, Lassmann H, Frahm J, Brück W (1999) Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR 20:1619–1627
Fazekas F, Kleinert R, Offenbacher H (1991) The morphologic correlate of incidental punctate white matter hyperintensities on MR images. AJNR Am J Neuroradiol 12:915–921
Scheltens P, Barkhof F, Leys D, Wolters E, Ravid R, Kamphorst W (1995) Histopathologic correlates of white matter changes on MRI in Alzheimer’s disease and normal aging. Neurol 45:883–888
Davie C, Barker G, Thompson A, Tofts P, McDonald W, Miller D (1997) 1H magnetic resonance spectroscopy of chronic cerebral white matter lesions and normal appearing white matter in multiple sclerosis. J Neurol Neurosurg Psychiatry 63:736–742
Van Walderveen M, Kamphorst W, Scheltens P, van Waesberghe J, Ravid R, Valk J, Polman C, Barkhof F (1998) Histopathologic correlate of hypointense lesions of T1–weighted spinecho MRI in multiple sclerosis. Neurology 50:1282–1288
Brex P, Gomez–Anson B, Parker G, Molyneux P, Miszkiel K, Barker G, MacManus D, Davie C, Plant G, DH M (1999) Proton MR spectroscopy in clinically isolated syndromes suggestive of multiple sclerosis. J Neurol Sci 166:16–22
Brex P, Parker G, Leary S, Molyneux P, Barker G, Davie C, Thompson A, Miller D (2000) Lesion heterogeneity in multiple sclerosis: a study of the relations between appearances on T1 weighted images, T1 relaxation times, and metabolite concentrations. J Neurol Neurosurg Psychiatry 68:627–632
Kapeller P, McLean M, Griffin C, Chard D, Parker G, Barker G, Thompson A, Miller D (2001) Evidence for neuronal damage in cortical grey matter and normal appearing white matter in early relapsing remitting multiple sclerosis: a quantitative MR spectroscopic imaging study. J Neurol 248:131–138
Kapeller P, Brex P, Chard D, Dalton C, Griffin C, McLean M, Parker G, Thompson A, Miller D (2002) Quantitative 1H MRS imaging 14 years after presenting with a clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler 8:207–210
Miller B, Moats R, Shonk T, Ernst T, Wooley S, Ross B (1993) Alzheimer disease: depiction of increased cerebral myo–inositol with proton MR Spectrosc. Radiol 187:433–437
Behar K, Boucher R, Fritch W, Manuelidis L (1998) Changes in N–acetylaspartate and myo–inositol detected in the cerebral cortex of hamsters with Creutzfeld–Jakob disease. MRI 16:963–968
Thurston J, Sherman W, Hauhart R, Kloepper R (1989) myo–inositol: a newly identified nonnitrogenous osmoregulatory molecule in mammalian brain. Pediatr Res 26:482–485
Chard D, Griffin C, McLean M, Kapeller P, Kapoor R, Thompson A, Miller D (2002) Brain metabolite changes in cortical grey and normalappearing white matter in clinically early relapsing–remitting multiple sclerosis. Brain 125:2342–2352
Oppenheimer S, Bryan R, Conturo T, Soher B, Preziosi T, Barker P (1995) Proton magnetic resonance spectroscopy and gadolinium–DTPA perfusion imaging of asymptomatic MRI white matter lesions. Magn Reson Med 33:61–68
Provencher S (1993) Estimation of metabolite concentrations from localised in vivo proton NMR spectra. MRM 30:672–679
Brand A, Richter–Landsberg C, Leibfritz D (1993) Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci 15:289–298
Simmons M, Frondoza C, Coyle J (1991) Immunocytochemical localisation of N–acetyl–aspartate with monoclonal antibodies. Neuroscience 45:37–45
Narayana P, Doyle T, Lai D, Wolinsky J (1998) Serial proton magnetic resonance spectroscopic imaging, contrast enhanced magnetic resonance imaging, and quantitative lesion volumetry in multiple sclerosis. Ann Neurol 43:56–71
Gonen O, Catalaa I, Babb J, Ge Y, Mannon L, Kolson D, Grossman R (2000) Total brain N–acetylaspartate: a new measure of disease load in MS. Neurology Jan 11:15–19
Van Waesberghe J, Kamphorst W, de Groot C, van Walderveen M, Castelijns J, Ravid R, Lycklama a Nijeholt G, van der Valk P, Polman C, Thompson A, Barkhof F (1999) Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol 46:747–754
Buniatian HC, Hovhannissian VS, Aprikian GV (1965) The Participation of N–Acetyl–l–Aspartic acid in Brain Metabolism. J Neurochem 12:695–703
Awad I, Spetzler R, Hodak J, Awad C, Carey R (1986) Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. I. Correlation with age and cerebrovascular risk factors. Stroke 17:1084–1089
Schmidt R, Fazekas F, Hayn M, Schmidt H, Kapeller P, Roob G, Offenbacher H, Schumacher M, Eber B, Weinrauch V, Kostner G, Esterbauer H (1997) Risk factors for microangiopathy– related cerebral damage in the Austrian stroke prevention study. J Neurol Sci 152:15–21
Schmidt R, Fazekas F, Offenbacher H, Kapeller P, Schmidt H, Roob G (1998) Prevalence and risk factors for white matter damage. In: Fazekas F, Schmidt R, Alavi A (eds) Neuroimaging of normal ageing and uncommon causes of dementia. Dordrecht: ICG Publications, pp 11–25
31. Kapeller P, Fazekas F, Schmidt R (2002) Vascular white matter changes. In: Leys D (ed) Stroke: Predisposing Conditions. London: Remedica, pp 89–97
Fazekas F (1989) Magnetic resonance signal abnormalities in asymptomatic individuals: their incidence and functional correlates. Eur Neurol 29:164–168
Mader I, Roser W, Kappos L, Hagberg G, Seelig J, Radue E, Steinbrich W (2000) Serial Proton MR Spectroscopy of Contrast–enhancing Multiple Sclerosis Plaques: Absolute Metabolic Values over 2 Years during a Clinical Pharmacological Study. AJNR 21:1220–1227
Inglese M, Li B, Rusinek H, Babb J, Grossman R, Gonen O (2003) Diffusely elevated cerebral choline and creatine in relapsing–remitting multiple sclerosis. Magn Reson Med 50:190–195
Van Walderveen M, Barkhof F, Pouwels P, van Schijndel R, Polman C, Castelijns J (1999) Neuronal damage in T1– hypointense multiple sclerosis lesions demonstrated in vivo using 1H MR spectroscopy. Ann Neurol 46:79–87
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kapeller, P., Ropele, S., Enzinger, C. et al. Discrimination of white matter lesions and multiple sclerosis plaques by short echo quantitative 1H—magnetic resonance spectroscopy. J Neurol 252, 1229–1234 (2005). https://doi.org/10.1007/s00415-005-0847-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-005-0847-3