Abstract
Objective
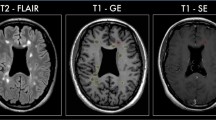
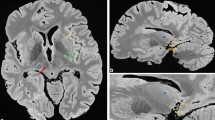
Cortical lesions in multiple sclerosis (MS) are notoriously difficult to visualize with standard MR imaging (MRI) techniques. However, the use of higher field-strengths with intrinsically higher signal-to-noise, which can partly be used to increase spatial resolution, may improve cortical lesion detection. Therefore, in this post mortem study, the sensitivity of high fieldstrength MRI (4.7 T) for cortical lesions was investigated, and compared to that of standard field-strength (1.5 T).
Methods
At 1.5 T, dual-echo T2-weighted spin-echo, as well as 3D-FLAIR images of seventeen formalin-fixed coronal MS and four control hemispheres were acquired. At 4.7 T, the same specimens were imaged with a mainly proton-density (PD)- weighted sequence. Proteolipid protein (PLP)-stained tissue sections (10 μm) of the same brain slices were matched to the corresponding MR images, and cortical lesions were scored on all three MR sequences (blinded to histology) and in tissue sections (blinded to MRI). Sensitivity of the sequences for four cortical lesion types was calculated. Additionally, an unblinded, retrospective MR scoring was performed.
Results
Sensitivity for purely intracortical lesions (histological lesion types II, III, and IV; n = 128) was below 10 % for both 1.5 T and 4.7 T MRI, while mixed gray matter-white matter (type I) lesions (n = 5) were detected in four out of five cases. All lesion counts increased upon retrospective (unblinded) scoring. However, up to 80% of the intracortical lesions still remained undetected.
Conclusions
MRI sensitivity for post mortem detection of cortical lesions is low, even when a higher field-strength was used. It varies, however, for different subtypes of cortical lesions.
Similar content being viewed by others
References
Barkhof F (2002) The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol 15:239–245
Barkhof F, Filippi M, Miller DH, Scheltens P, Campi A, Polman CH, Comi G, Ader HJ, Losseff N, Valk J (1997) Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 120:2059–2069
Benedict RH, Zivadinov R, Carone DA, Weinstock-Guttman B, Gaines J, Maggiore C, Sharma J, Tomassi MA, Bakshi R (2005) Regional lobar atrophy predicts memory impairment in multiple sclerosis. AJNR Am J Neuroradiol 26:1824–1831
Blamire AM, Rowe JG, Styles P, McDonald B (1999) Optimising imaging parameters for post mortem MR imaging of the human brain. Acta Radiol 40:593–597
Bo L, Geurts JJ, Mork SJ, van der Valk P (2006) Grey matter pathology in multiple sclerosis. Acta Neurol Scand Suppl 183:48–50
Bo L, Geurts JJ, Ravid R, Barkhof F (2004) Magnetic resonance imaging as a tool to examine the neuropathology of multiple sclerosis. Neuropathol Appl Neurobiol 30:106–117
Bo L, Geurts JJ, van der Valk P, Polman C, Barkhof F (2007) Lack of correlation between cortical demyelination and white matter pathologic changes in multiple sclerosis. Arch Neurol 64:76–80
Bo L, Vedeler CA, Nyland H, Trapp BD, Mork SJ (2003) Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler 9:323–331
Bo L, Vedeler CA, Nyland HI, Trapp BD, Mork SJ (2003) Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol 62:723–732
Bozzali M, Cercignani M, Sormani MP, Comi G, Filippi M (2002) Quantification of brain gray matter damage in different MS phenotypes by use of diffusion tensor MR imaging. AJNR Am J Neuroradiol 23:985–988
Brink BP, Veerhuis R, Breij EC, van der Valk P, Dijkstra CD, Bo L (2005) The pathology of multiple sclerosis is location-dependent: no significant complement activation is detected in purely cortical lesions. J Neuropathol Exp Neurol 64:147–155
Brownell B and Hughes JT (1962) The distribution of plaques in the cerebrum in multiple sclerosis. J Neurol Neurosurg Psychiatry 25:315–320
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M (2001) Magnetisation transfer ratio and mean diffusivity of normal appearing white and grey matter from patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 70:311–317
Dalton CM, Chard DT, Davies GR, Miszkiel KA, Altmann DR, Fernando K, Plant GT, Thompson AJ, Miller DH (2004) Early development of multiple sclerosis is associated with progressive grey matter atrophy in patients presenting with clinically isolated syndromes. Brain 127:1101–1107
Davies GR, Ramio-Torrenta L, Hadjiprocopis A, Chard DT, Griffin CM, Rashid W, Barker GJ, Kapoor R, Thompson AJ, Miller DH (2004) Evidence for grey matter MTR abnormality in minimally disabled patients with early relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 75:998–1002
Dawson JW (1916) The Histology of Multiple Sclerosis. Trans R Soc Edinburgh 50:517–740
De Groot CJ, Bergers E, Kamphorst W, Ravid R, Polman CH, Barkhof F, van der Valk P (2001) Post-mortem MRI-guided sampling of multiple sclerosis brain lesions: increased yield of active demyelinating and (p)reactive lesions. Brain 124:1635–1645
De Stefano N, Matthews PM, Filippi M, Agosta F, De Luca M, Bartolozzi ML, Guidi L, Ghezzi A, Montanari E, Cifelli A, Federico A, Smith SM (2003) Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology 60:1157–1162
Feinstein A, Roy P, Lobaugh N, Feinstein K, O'Connor P, Black S (2004) Structural brain abnormalities in multiple sclerosis patients with major depression. Neurology 62:586–590
Ge Y, Grossman RI, Udupa JK, Babb JS, Kolson DL, McGowan JC (2001) Magnetization transfer ratio histogram analysis of gray matter in relapsing-remitting multiple sclerosis. AJNR Am J Neuroradiol 22:470–475
Geurts JJ, Bo L, Pouwels PJ, Castelijns JA, Polman CH, Barkhof F (2005) Cortical lesions in multiple sclerosis: combined postmortem MR imaging and histopathology. AJNR Am J Neuroradiol 26:572–577
Geurts JJ, Pouwels PJ, Uitdehaag BM, Polman CH, Barkhof F, Castelijns JA (2005) Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging. Radiology 236:254–260
Henkelman RM (1985) Measurement of signal intensities in the presence of noise in MR images. Med Phys 12:232–233
Kidd D, Barkhof F, McConnell R, Algra PR, Allen IV, Revesz T (1999) Cortical lesions in multiple sclerosis. Brain 122:17–26
Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H (2005) Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128:2705–2712
Lazeron RH, Langdon DW, Filippi M, van Waesberghe JH, Stevenson VL, Boringa JB, Origgi D, Thompson AJ, Falautano M, Polman CH, Barkhof F (2000) Neuropsychological impairment in multiple sclerosis patients: the role of (juxta)cortical lesion on FLAIR. Mult Scler 6:280–285
Macchi G and Cioffi RP (1992) An in vivo and post mortem MRI study in multiple sclerosis with pathological correlation. Ital J Neurol Sci 13:97–103
McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 50:121–127
Moriarty DM, Blackshaw AJ, Talbot PR, Griffiths HL, Snowden JS, Hillier VF, Capener S, Laitt RD, Jackson A (1999) Memory dysfunction in multiple sclerosis corresponds to juxtacortical lesion load on fast fluid-attenuated inversion-recovery MR images. AJNR Am J Neuroradiol 20:1956–1962
Nagara H, Inoue T, Koga T, Kitaguchi T, Tateishi J, Goto I (1987) Formalin fixed brains are useful for magnetic resonance imaging (MRI) study. J Neurol Sci 81:67–77
Peterson JW, Bo L, Mork S, Chang A, Trapp BD (2001) Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 50:389–400
Rovaris M, Filippi M, Minicucci L, Iannucci G, Santuccio G, Possa F, Comi G (2000) Cortical/subcortical disease burden and cognitive impairment in patients with multiple sclerosis. AJNR Am J Neuroradiol 21:402–408
Schmierer K, Scaravilli F, Altmann DR, Barker GJ, Miller DH (2004) Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol 56:407–415
Sokic DV, Stojsavljevic N, Drulovic J, Dujmovic I, Mesaros S, Ercegovac M, Peric V, Dragutinovic G, Levic Z (2001) Seizures in multiple sclerosis. Epilepsia 42:72–79
Vercellino M, Plano F, Votta B, Mutani R, Giordana MT, Cavalla P (2005) Grey matter pathology in multiple sclerosis. J Neuropathol Exp Neurol 64:1101–1107
Vos CM, Geurts JJ, Montagne L, van Haastert ES, Bo L, van der Valk P, Barkhof F, de Vries HE (2005) Blood-brain barrier alterations in both focal and diffuse abnormalities on postmortem MRI in multiple sclerosis. Neurobiol Dis 20:953–960
Vrenken H, Pouwels PJ, Geurts JJ, Knol DL, Polman CH, Barkhof F, Castelijns JA (2006) Altered diffusion tensor in multiple sclerosis normal-appearing brain tissue: cortical diffusion changes seem related to clinical deterioration. J Magn Reson Imaging 23:628–636
Wegner C, Esiri MM, Chance SA, Palace J, Matthews PM (2006) Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology 67:960–967
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geurts, J.J.G., Blezer, E.L.A., Vrenken, H. et al. Does high-field MR imaging improve cortical lesion detection in multiple sclerosis?. J Neurol 255, 183–191 (2008). https://doi.org/10.1007/s00415-008-0620-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-008-0620-5