Abstract
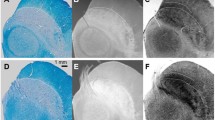
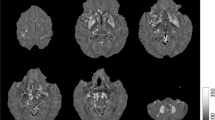
Iron plays an important role in many neurobiological processes, especially in the basal ganglia, the brain structures with the highest concentration. Composed of the pallidum and putamen, the lentiform nucleus plays a key role in the basal ganglia circuitry. With MRI advances, iron-based sequences such as R2* and quantitative susceptibility mapping (QSM) are now available for detecting and quantifying iron in different brain structures. Since their validation using classic iron detection techniques (histology or physical techniques), these sequences have attracted growing clinical attention, especially in the field of extrapyramidal syndromes that particularly affect the basal nuclei. Accurate mapping of iron in these nuclei and their connections is needed to gain a better understanding of this specific anatomy, before considering its involvement in the physiopathological processes. We performed R2* and QSM along with Perls histology, to gain new insights into the distribution of iron in the lentiform nucleus and its surrounding structures, based on four specimens obtained from voluntary donors. We found that iron is preferentially distributed in the anterior part of the globus pallidus externus and the posterior part of the putamen. The lateral wall of the putamen is iron-poor, compared with the lateral medullary lamina and intraputaminal fibers. The relevance of perivascular iron concentration, along with pallido- and putaminofugal iron-rich fibers, is discussed.








Similar content being viewed by others
Availability of data and materials
MRI and histological images are available asking to first author.
References
Alho EJL, Alho ATDL, Horn A, da Martin M, GM, Edlow BL, Fischl B, et al (2019) The ansa subthalamica: a neglected fiber tract. Movem Dis. https://doi.org/10.1002/mds.27901
Bacyinski A, Xu M, Wang W, Hu J (2017) The paravascular pathway for brain waste clearance: current understanding, significance and controversy. Front Neuroanat. https://doi.org/10.3389/fnana.2017.00101/full
Barbagallo G, Sierra-Peña M, Nemmi F, Traon AP-L, Meissner WG, Rascol O et al (2016) Multimodal MRI assessment of nigro-striatal pathway in multiple system atrophy and Parkinson disease. Mov Disord 31(3):325–34
Chen L, Cai C, Yang T, Lin J, Cai S, Zhang J et al (2017) Changes in brain iron concentration after exposure to high-altitude hypoxia measured by quantitative susceptibility mapping. Neuroimage 147:488–99
Daugherty AM, Raz N (2016) Accumulation of iron in the putamen predicts its shrinkage in healthy older adults: a multi-occasion longitudinal study. Neuromage 128:11–20
De Barros A, Arribarat G, Combis J, Chaynes P, Péran P (2019) Matching ex vivo MRI with iron histology: pearls and pitfalls. Front Neuroanat. https://doi.org/10.3389/fnana.2019.00068/full
de Hollander G, Keuken MC, Bazin P-L, Weiss M, Neumann J, Reimann K et al (2014) A gradual increase of iron toward the medial-inferior tip of the subthalamic nucleus. Hum Brain Mapp 35(9):4440–9
de Rochefort L, Liu T, Kressler B, Liu J, Spincemaille P, Lebon V et al (2010) Quantitative susceptibility map reconstruction from MR phase data using Bayesian regularization: validation and application to brain imaging. Magn Reson Med 63(1):194–206
Dormont D, Ricciardi KG, Tandé D, Parain K, Menuel C, Galanaud D et al (2004) Is the subthalamic nucleus hypointense on T2-weighted images? A correlation study using MR imaging and stereotactic atlas data. AJNR Am J Neuroradiol 25(9):1516–23
Drayer B, Burger P, Darwin R, Riederer S, Herfkens R, Johnson GA (1986) MRI of brain iron. Am J Roentgenol. https://doi.org/10.2214/ajr.147.1.103
Dusek P, Jankovic J, Le W (2012) Iron dysregulation in movement disorders. Neurobiol Dis 46(1):1–18
Duyn JH (2017) Studying brain microstructure with magnetic susceptibility contrast at high-field. Neuroimage http://www.sciencedirect.com/science/article/pii/S105381191730157X
Fisher M, French S, Ji P, Kim RC (2010) Cerebral microbleeds in the elderly: a pathological analysis. Stroke 41(12):2782–5
Fujii S, Matsusue E, Kinoshita T, Sugihara S, Ohama E, Ogawa T (2007) Hyperintense putaminal rim at 3T reflects fewer ferritin deposits in the lateral marginal area of the putamen. Am J Neuroradiol 28(4):777–81
Gray MT, Woulfe JM (2015) Striatal blood–brain barrier permeability in Parkinson’s disease. J Cereb Blood Flow Metab 35(5):747–50
Haacke EM, Cheng NYC, House MJ, Liu Q, Neelavalli J, Ogg RJ et al (2005) Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 23(1):1–25
Haber SN (2016) Corticostriatal circuitry. Dial Clin Neurosci 18(1):15
Hallgren B, Sourander P (1958) The effect of age on the non-haemin iron in the human brain. J Neurochem 3(1):41–51
Hametner S, Endmayr V, Deistung A, Palmrich P, Prihoda M, Haimburger E et al (2018) The influence of brain iron and myelin on magnetic susceptibility and effective transverse relaxation - A biochemical and histological validation study. Neuroimage 179:117–33
Hoch MJ, Bruno MT, Faustin A, Cruz N, Mogilner AY, Crandall L et al (2019) 3T MRI whole-brain microscopy discrimination of subcortical anatomy, part 2: basal forebrain. Am J Neuroradiol 40(7):1095–105
Janaway BM, Simpson JE, Hoggard N, Highley JR, Forster G, Drew D et al (2014) Brain haemosiderin in older people: pathological evidence for an ischaemic origin of magnetic resonance imaging (MRI) microbleeds. Neuropathol Appl Neurobiol 40(3):258–69
Kaindlstorfer C, Jellinger KA, Eschlböck S, Stefanova N, Weiss G, Wenning GK (2018) The relevance of iron in the pathogenesis of multiple system atrophy: a viewpoint. J Alzheimers Dis 61(4):1253–73
Lang AE, Curran T, Provias J, Bergeron C (1994) Striatonigral degeneration: iron deposition in putamen correlates with the slit-like void signal of magnetic resonance imaging. Canadian J Neurol Sci 21(4):311–8
Langkammer C, Krebs N, Goessler W, Scheurer E, Ebner F, Yen K et al (2010) Quantitative MR imaging of brain iron: a postmortem validation study. Radiology 257(2):455–62
Lehéricy S, Ducros M, Van De Moortele P-F, Francois C, Thivard L, Poupon C et al (2004) Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol 55(4):522–9
Li W, Avram AV, Wu B, Xiao X, Liu C (2014) Integrated Laplacian-based phase unwrapping and background phase removal for quantitative susceptibility mapping. NMR Biomed 27(2):219–227. https://doi.org/10.1002/nbm.3056
Li W, Liu C, Duong TQ, van Zijl PCM, Li X (2017) Susceptibility tensor imaging (STI) of the brain. NMR Biomed 30(4).
Liu J, Liu T, de Rochefort L, Ledoux J, Khalidov I, Chen W et al (2012) Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage 59(3):2560–8
Loureiro JR, Himmelbach M, Ethofer T, Pohmann R, Martin P, Bause J et al (2018) In-vivo quantitative structural imaging of the human midbrain and the superior colliculus at 9.4T. Neuroimage 177:117–28
Martin WRW, Wieler M, Gee M (2008) Midbrain iron content in early Parkinson disease: a potential biomarker of disease status. Neurology 70(16 Pt 2):1411–7
Matsusue E, Fujii S, Kanasaki Y, Sugihara S, Miyata H, Ohama E et al (2008) Putaminal lesion in multiple system atrophy: postmortem MR-pathological correlations. Neuroradiology 50(7):559–67
Morris CM, Candy JM, Oakley AE, Bloxham CA, Edwardson JA (1992) Histochemical distribution of non-haem iron in the human brain. Acta Anat 144(3):235–57
Nauta WJ, Mehler WR (1993) The lentiform nucleus in the monkey. In: Projections of the Lentiform Nucleus in the Monkey (ed.) Neuroanatomy. Birkhäuser, Boston. http://public.ebookcentral.proquest.com/choice/publicfullrecord.aspx?p=3084147
Ndayisaba A, Kaindlstorfer C, Wenning GK (2019) Iron in neurodegeneration - cause or consequence? Front Neurosci 13:180
Péran P, Hagberg G, Luccichenti G, Cherubini A, Brainovich V, Celsis P et al (2007) Voxel-based analysis of R2* maps in the healthy human brain. J Magn Reson Imaging 26(6):1413–20
Péran P, Cherubini A, Luccichenti G, Hagberg G, Démonet J-F, Rascol O et al (2009) Volume and iron content in basal ganglia and thalamus. Hum Brain Mapp 30(8):2667–75
Péran P, Barbagallo G, Nemmi F, Sierra M, Galitzky M, Traon AP-L et al (2018) MRI supervised and unsupervised classification of Parkinson’s disease and multiple system atrophy. Mov Disord 33(4):600–8
Plantinga BR, Roebroeck A, Kemper VG, Uludağ K, Melse M, Mai J et al (2016) Ultra-high field MRI post mortem structural connectivity of the human subthalamic nucleus, substantia nigra, and globus pallidus. Front Neuroanat 10. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4909758/
Ribas EC, Yağmurlu K, de Oliveira E, Ribas GC, Rhoton A (2018) Microsurgical anatomy of the central core of the brain. J Neurosurg 129(3):752–69
Rutledge J, Hilal S, Silver A, Defendini R, Fahn S (1987) Study of movement disorders and brain iron by MR. Am J Roentgenol 149(2):365–79
Schneider TM, Deistung A, Biedermann U, Matthies C, Ernestus R-I, Volkmann J et al (2016) Susceptibility sensitive magnetic resonance imaging displays pallidofugal and striatonigral fiber tracts: Operative. Neurosurgery 12(4):330–8
Schweser F, Deistung A, Lehr BW, Reichenbach JR (2011) Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism? Neuroimage 54(4):2789–807
Stockwell BR, Angeli JPF, Bayir H, Bush AI, Conrad M, Dixon S et al (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171(2):273–85
Sun H, Walsh AJ, Lebel RM, Blevins G, Catz I, Lu J-Q et al (2015) Validation of quantitative susceptibility mapping with Perls’ iron staining for subcortical gray matter. Neuroimage 105:486–92
Tha KK, Terae S, Tsukahara A, Soma H, Morita R, Yabe I et al (2012) Hyperintense putaminal rim at 1.5 T: prevalence in normal subjects and distinguishing features from multiple system atrophy. BMC Neurol 12:39
Vymazal J, Brooks RA, Baumgarner C, Tran V, Katz D, Bulte JW et al (1996) The relation between brain iron and NMR relaxation times: an in vitro study. Magn Reson Med 35(1):56–61
Wallis LI, Paley MNJ, Graham JM, Grünewald RA, Wignall EL, Joy HM et al (2008) MRI assessment of basal ganglia iron deposition in Parkinson’s disease. J Magn Reson Imaging 28(5):1061–7
Walsh AJ, Lebel RM, Eissa A, Blevins G, Catz I, Lu J-Q et al (2013) Multiple sclerosis: validation of MR imaging for quantification and detection of iron. Radiology 267(2):531–42
Wang N, Yang H, Li C, Fan G, Luo X (2017) Using “swallow-tail” sign and putaminal hypointensity as biomarkers to distinguish multiple system atrophy from idiopathic Parkinson’s disease: a susceptibility-weighted imaging study. Eur Radiol 27(8):3174–80
Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L (2014) The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13(10):1045–60
Watanabe H, Ito M, Fukatsu H, Senda J, Atsuta N, Kaga T et al (2010) Putaminal magnetic resonance imaging features at various magnetic field strengths in multiple system atrophy. Movem Dis 25(12):1916–23
Wolfram-Gabel R, Maillot C (1994) Vascular networks of the nucleus lentiformis. Surg Radiol Anat 16(4):373–7
Wolfram-Gabel R, Maillot C (1995) Arterial vascularization of the lenticular nucleus. J Neuroradiol 22(1):1–11
Zheng W, Nichol H, Liu S, Cheng Y-CN, Haacke EM (2013) Measuring iron in the brain using quantitative susceptibility mapping and X-ray fluorescence imaging. Neuroimage 78:68–74
Funding
Funding was supported by Fondation pour la recherche médicale (FRM); Grant numbers: DEA20170839104.
Author information
Authors and Affiliations
Contributions
ADB, JAL, PC, and PP conceived the protocol, ADB and PC performed dissection, ADB wrote the manuscript, GA and PP performed MRI, GD performed histology, and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
In accordance with Article 16 of the Civil Code on the Respect of the Human Body and Article 3 of the Law of 15 November 1887 on the Freedom of Funerals, each donor, during his or her lifetime, expressed in a written and signed manner his or her willingness to donate his or her body to science. The originals are kept in the anatomy laboratory.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
De Barros, A., Arribarat, G., Lotterie, J.A. et al. Iron distribution in the lentiform nucleus: A post-mortem MRI and histology study. Brain Struct Funct 226, 351–364 (2021). https://doi.org/10.1007/s00429-020-02175-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-020-02175-7