Abstract
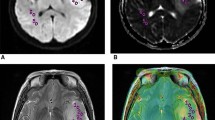
Tumor grade and molecular variants influence the survival of patients with glioma. The apparent diffusion coefficient (ADC) map is a non-invasive tool for evaluating the outcomes and response to therapy in glioma. In this study, we investigated the correlation between the tumor grade and prognostic biomarkers with the ADC in glioma patients. Eighty-two patients with supratentorial glioma were identified via analysis of surgical specimens and neuroradiological data. Using the World Health Organization grade, histological subtype, and molecular variants (1p/19q codeletion, isocitrate dehydrogenase 1/2 mutation, Ki-67 index, O6-methylguanine DNA methyltransferase, P53, and vascular endothelial growth factor immunoactivity) as prognostic biomarkers, we performed receiver operating characteristic analysis and multiple linear regression to assess the association between the magnetic resonance diffusion parameter and mean ADC and the prognostic factors of glioma pathology. Univariate analysis and multiple linear regression revealed inverse correlations between the ADC values and the tumor grade, oligodendrocytoma histology, and 1p/19q codeletion. A threshold mean ADC value could predict the 1p/19q chromosomal status in WHO II gliomas with 72 % sensitivity and 88 % specificity (area under the curve 0.82, 95 % confidence interval 0.68–0.97) and could distinguish low-grade glioma with low-risk factors from the high-risk group (P < 0.01). The mean ADC value could be used as a non-invasive tool to evaluate the prognosis of supratentorial glioma patients. A threshold mean ADC value could be used to predict the 1p/19q codeletion and to identify low-risk low-grade gliomas (LGGs). Lower ADC values are indicative of a favorable prognosis in LGGs.



Similar content being viewed by others
References
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. doi:10.1007/s00401-007-0243-4
Hoang-Xuan K, Capelle L, Kujas M, Taillibert S, Duffau H, Lejeune J, Polivka M, Criniere E, Marie Y, Mokhtari K, Carpentier AF, Laigle F, Simon JM, Cornu P, Broet P, Sanson M, Delattre JY (2004) Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol 22:3133–3138. doi:10.1200/JCO.2004.10.169
Kaloshi G, Benouaich-Amiel A, Diakite F, Taillibert S, Lejeune J, Laigle-Donadey F, Renard MA, Iraqi W, Idbaih A, Paris S, Capelle L, Duffau H, Cornu P, Simon JM, Mokhtari K, Polivka M, Omuro A, Carpentier A, Sanson M, Delattre JY, Hoang-Xuan K (2007) Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology 68:1831–1836. doi:10.1212/01.wnl.0000262034.26310.a2
Cairncross G, Macdonald D, Ludwin S, Lee D, Cascino T, Buckner J, Fulton D, Dropcho E, Stewart D, Schold C Jr (1994) Chemotherapy for anaplastic oligodendroglioma. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 12:2013–2021
Ino Y, Betensky RA, Zlatescu MC, Sasaki H, Macdonald DR, Stemmer-Rachamimov AO, Ramsay DA, Cairncross JG, Louis DN (2001) Molecular subtypes of anaplastic oligodendroglioma: implications for patient management at diagnosis. Clin Cancer Res 7:839–845
van den Bent M, Chinot OL, Cairncross JG (2003) Recent developments in the molecular characterization and treatment of oligodendroglial tumors. Neuro Oncol 5:128–138. doi:10.1215/S1522-8517-02-00028-5
Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, El Hallani S, Boisselier B, Mokhtari K, Hoang-Xuan K, Delattre JY (2009) Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol 27:4150–4154. doi:10.1200/JCO.2009.21.9832
Houillier C, Wang X, Kaloshi G, Mokhtari K, Guillevin R, Laffaire J, Paris S, Boisselier B, Idbaih A, Laigle-Donadey F, Hoang-Xuan K, Sanson M, Delattre JY (2010) IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology 75:1560–1566. doi:10.1212/WNL.0b013e3181f96282
Pignatti F, van den Bent M, Curran D, Debruyne C, Sylvester R, Therasse P, Afra D, Cornu P, Bolla M, Vecht C, Karim AB (2002) Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol 20:2076–2084. doi:10.1200/JCO.2002.08.121
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. doi:10.1056/NEJMoa043330
Hegi ME, Diserens AC, Godard S, Dietrich PY, Regli L, Ostermann S, Otten P, Van Melle G, de Tribolet N, Stupp R (2004) Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res 10:1871–1874. doi:10.1158/1078-0432.CCR-03-0384
Hamstra DA, Chenevert TL, Moffat BA, Johnson TD, Meyer CR, Mukherji SK, Quint DJ, Gebarski SS, Fan X, Tsien CI, Lawrence TS, Junck L, Rehemtulla A, Ross BD (2005) Evaluation of the functional diffusion map as an early biomarker of time-to-progression and overall survival in high-grade glioma. Proc Natl Acad Sci USA 102:16759–16764. doi:10.1073/pnas.0508347102
Hamstra DA, Galban CJ, Meyer CR, Johnson TD, Sundgren PC, Tsien C, Lawrence TS, Junck L, Ross DJ, Rehemtulla A, Ross BD, Chenevert TL (2008) Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J Clin Oncol 26:3387–3394. doi:10.1200/JCO.2007.15.2363
Chang L, McBride D, Miller BL, Cornford M, Booth RA, Buchthal SD, Ernst TM, Jenden D (1995) Localized in vivo 1H magnetic resonance spectroscopy and in vitro analyses of heterogeneous brain tumors. J Neuroimaging 5:157–163
Chenevert TL, Stegman LD, Taylor JM, Robertson PL, Greenberg HS, Rehemtulla A, Ross BD (2000) Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst 92:2029–2036. doi:10.1093/jnci/92.24.2029
Sugahara T, Korogi Y, Kochi M, Ikushima I, Shigematu Y, Hirai T, Okuda T, Liang L, Ge Y, Komohara Y, Ushio Y, Takahashi M (1999) Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 9:53–60. doi:10.1002/(SICI)1522-2586(199901)9:1<53:AID-JMRI7>3.0.CO;2-2
Khayal IS, Vandenberg SR, Smith KJ, Cloyd CP, Chang SM, Cha S, Nelson SJ, McKnight TR (2011) MRI apparent diffusion coefficient reflects histopathologic subtype, axonal disruption, and tumor fraction in diffuse-type grade II gliomas. Neuro Oncol 13:1192–1201. doi:10.1093/neuonc/nor122
Gupta RK, Cloughesy TF, Sinha U, Garakian J, Lazareff J, Rubino G, Rubino L, Becker DP, Vinters HV, Alger JR (2000) Relationships between choline magnetic resonance spectroscopy, apparent diffusion coefficient and quantitative histopathology in human glioma. J Neurooncol 50:215–226. doi:10.1023/A:1006431120031
Kono K, Inoue Y, Nakayama K, Shakudo M, Morino M, Ohata K, Wakasa K, Yamada R (2001) The role of diffusion-weighted imaging in patients with brain tumors. Am J Neuroradiol 22:1081–1088
Higano S, Yun X, Kumabe T, Watanabe M, Mugikura S, Umetsu A, Sato A, Yamada T, Takahashi S (2006) Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology 241:839–846. doi:10.1148/radiol.2413051276
Murakami R, Sugahara T, Nakamura H, Hirai T, Kitajima M, Hayashida Y, Baba Y, Oya N, Kuratsu J, Yamashita Y (2007) Malignant supratentorial astrocytoma treated with postoperative radiation therapy: prognostic value of pretreatment quantitative diffusion-weighted MR imaging. Radiology 243:493–499. doi:10.1148/radiol.2432060450
Yamasaki F, Sugiyama K, Ohtaki M, Takeshima Y, Abe N, Akiyama Y, Takaba J, Amatya VJ, Saito T, Kajiwara Y, Hanaya R, Kurisu K (2010) Glioblastoma treated with postoperative radio-chemotherapy: prognostic value of apparent diffusion coefficient at MR imaging. Eur J Radiol 73:532–537. doi:10.1016/j.ejrad.2009.01.013
Zulfiqar M, Yousem DM, Lai H (2013) ADC values and prognosis of malignant astrocytomas: does lower ADC predict a worse prognosis independent of grade of tumor?–a meta-analysis. Am J Roentgenol 200:624–629. doi:10.2214/AJR.12.8679
Brasil Caseiras G, Ciccarelli O, Altmann DR, Benton CE, Tozer DJ, Tofts PS, Yousry TA, Rees J, Waldman AD, Jager HR (2009) Low-grade gliomas: six-month tumor growth predicts patient outcome better than admission tumor volume, relative cerebral blood volume, and apparent diffusion coefficient. Radiology 253:505–512. doi:10.1148/radiol.2532081623
National Comprehensive Cancer Network (2013) NCCN Guidelines® updates. J Natl Compr Canc Netw 11:1153–1164
Ren X, Cui X, Lin S, Wang J, Jiang Z, Sui D, Li J, Wang Z (2012) Co-deletion of chromosome 1p/19q and IDH1/2 mutation in glioma subsets of brain tumors in Chinese patients. PLoS One 7:e32764. doi:10.1371/journal.pone.0032764
Ambros PF, Ambros IM (2001) Pathology and biology guidelines for resectable and unresectable neuroblastic tumors and bone marrow examination guidelines. Med Pediatr Oncol 37:492–504. doi:10.1002/mpo.1242
Jiang H, Ren X, Zhang W, Ma J, Sui D, Jiang Z, Cui X, Lin S (2013) A new prognostic scoring scale for patients with primary WHO grade III gliomas based on molecular predictors. J Neurooncol 111:367–375. doi:10.1007/s11060-012-1026-x
Daniels TB, Brown PD, Felten SJ, Wu W, Buckner JC, Arusell RM, Curran WJ, Abrams RA, Schiff D, Shaw EG (2011) Validation of EORTC prognostic factors for adults with low-grade glioma: a report using intergroup 86-72-51. Int J Radiat Oncol Biol Phys 81:218–224. doi:10.1016/j.ijrobp.2010.05.003
Crawford FW, Khayal IS, McGue C, Saraswathy S, Pirzkall A, Cha S, Lamborn KR, Chang SM, Berger MS, Nelson SJ (2009) Relationship of pre-surgery metabolic and physiological MR imaging parameters to survival for patients with untreated GBM. J Neurooncol 91:337–351. doi:10.1007/s11060-008-9719-x
Ellingson BM, Malkin MG, Rand SD, Connelly JM, Quinsey C, LaViolette PS, Bedekar DP, Schmainda KM (2010) Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging 31:538–548. doi:10.1002/jmri.22068
Fellah S, Caudal D, De Paula AM, Dory-Lautrec P, Figarella-Branger D, Chinot O, Metellus P, Cozzone PJ, Confort-Gouny S, Ghattas B, Callot V, Girard N (2013) Multimodal MR imaging (diffusion, perfusion, and spectroscopy): is it possible to distinguish oligodendroglial tumor grade and 1p/19q codeletion in the pretherapeutic diagnosis? Am J Neuroradiol 34:1326–1333. doi:10.3174/ajnr.A3352
Pope WB, Qiao XJ, Kim HJ, Lai A, Nghiemphu P, Xue X, Ellingson BM, Schiff D, Aregawi D, Cha S, Puduvalli VK, Wu J, Yung WK, Young GS, Vredenburgh J, Barboriak D, Abrey LE, Mikkelsen T, Jain R, Paleologos NA, Rn PL, Prados M, Goldin J, Wen PY, Cloughesy T (2012) Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: a multi-center study. J Neurooncol 108:491–498. doi:10.1007/s11060-012-0847-y
Acknowledgments
The authors would like to thank Dr. Zhang Ming-Yu of the Department of Neuroimaging at Beijing Tiantan Hospital for his assistance with the image analysis and Dr. Ho Lok Yan of the Department of Neurosurgery at Princess Margaret Hospital in Hong Kong and Lian-Xiong Yuan of the Department of Statistics and Epidemiology, School of Public Health, at Sun Yat-Sen University for their assistance with the manuscript preparation and data analysis.
Conflict of interest
None.
Disclosure of funding
Beijing Natural Science Foundation (7122061 to Song Lin). No personal or institutional financial interest in drugs, materials, or devices described in our submission
Author information
Authors and Affiliations
Corresponding author
Additional information
Yong Cui and Li Ma have contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cui, Y., Ma, L., Chen, X. et al. Lower apparent diffusion coefficients indicate distinct prognosis in low-grade and high-grade glioma. J Neurooncol 119, 377–385 (2014). https://doi.org/10.1007/s11060-014-1490-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1490-6