Abstract
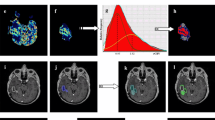
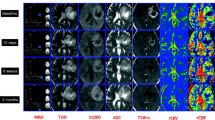
The goal of this study is to spatially discriminate tumor from treatment effect (TE), within the contrast-enhancing lesion, for brain tumor patients at all stages of treatment. To this end, the diagnostic accuracy of MRI-derived diffusion and perfusion parameters to distinguish pure TE from pure glioblastoma (GBM) was determined utilizing spatially-correlated biopsy samples. From July 2010 through June 2015, brain tumor patients who underwent pre-operative DWI and DSC-MRI and stereotactic image-guided biopsy were considered for inclusion in this IRB-approved study. MRI-derived parameter maps included apparent diffusion coefficient (ADC), normalized cerebral blood flow (nCBF), normalized and standardized relative cerebral blood volume (nRCBV, sRCBV), peak signal-height (PSR) and percent signal-recovery (PSR). These were co-registered to the Stealth MRI and median values extracted from the spatially-matched biopsy regions. A ROC analysis accounting for multiple subject samples was performed, and the optimal threshold for distinguishing TE from GBM determined for each parameter. Histopathologic diagnosis of pure TE (n = 10) or pure GBM (n = 34) was confirmed in tissue samples from 15 consecutive subjects with analyzable data. Perfusion thresholds of sRCBV (3575; SN/SP% = 79.4/90.0), nRCBV (1.13; SN/SP% = 82.1/90.0), and nCBF (1.05; SN/SP% = 79.4/80.0) distinguished TE from GBM (P < 0.05), whereas ADC, PSR, and PH could not (P > 0.05). The thresholds for CBF and CBV can be applied to lesions with any admixture of tumor or treatment effect, enabling the identification of true tumor burden within enhancing lesions. This approach overcomes current limitations of averaging values from both tumor and TE for quantitative assessments.




Similar content being viewed by others
References
Eisele SC, Wen PY, Lee EQ (2016) Assessment of brain tumor response: RANO and its offspring. Curr Treat Options Oncol 17:35. doi:10.1007/s11864-016-0413-5
Raimbault A, Cazals X, Lauvin MA, Destrieux C, Chapet S, Cottier JP (2014) Radionecrosis of malignant glioma and cerebral metastasis: a diagnostic challenge in MRI. Diagn Interv Imaging 95:985–1000. doi:10.1016/j.diii.2014.06.013
Verma N, Cowperthwaite MC, Burnett MG, Markey MK (2013) Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro Oncol 15:515–534. doi:10.1093/neuonc/nos307
Brandes AA, Tosoni A, Spagnolli F, Frezza G, Leonardi M, Calbucci F, Franceschi E (2008) Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol 10:361–367. doi:10.1215/15228517-2008-008
Parvez K, Parvez A, Zadeh G (2014) The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int J Mol Sci 15:11832–11846. doi:10.3390/ijms150711832
Barajas RF Jr, Chang JS, Segal MR, Parsa AT, McDermott MW, Berger MS, Cha S (2009) Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 253:486–496. doi:10.1148/radiol.2532090007
Gahramanov S, Muldoon LL, Varallyay CG, Li X, Kraemer DF, Fu R, Hamilton BE, Rooney WD, Neuwelt EA (2013) Pseudoprogression of glioblastoma after chemo- and radiation therapy: diagnosis by using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging with ferumoxytol versus gadoteridol and correlation with survival. Radiology 266:842–852. doi:10.1148/radiol.12111472
Nasseri M, Gahramanov S, Netto JP, Fu R, Muldoon LL, Varallyay C, Hamilton BE, Neuwelt EA (2014) Evaluation of pseudoprogression in patients with glioblastoma multiforme using dynamic magnetic resonance imaging with ferumoxytol calls RANO criteria into question. Neuro Oncol 16:1146–1154. doi:10.1093/neuonc/not328
Law M, Young RJ, Babb JS, Peccerelli N, Chheang S, Gruber ML, Miller DC, Golfinos JG, Zagzag D, Johnson G (2008) Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 247:490–498. doi:10.1148/radiol.2472070898
Al-Okaili RN, Krejza J, Woo JH, Wolf RL, O’Rourke DM, Judy KD, Poptani H, Melhem ER (2007) Intraaxial brain masses: MR imaging-based diagnostic strategy–initial experience. Radiology 243:539–550. doi:10.1148/radiol.2432060493
Khalifa J, Tensaouti F, Chaltiel L, Lotterie JA, Catalaa I, Sunyach MP, Ibarrola D, Noel G, Truc G, Walker P, Magne N, Charissoux M, Ken S, Peran P, Berry I, Moyal EC, Laprie A (2016) Identification of a candidate biomarker from perfusion MRI to anticipate glioblastoma progression after chemoradiation. Eur Radiol. doi:10.1007/s00330-016-4234-5
Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, Knopp EA, Zagzag D (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 24:1989–1998
Barajas RF Jr, Phillips JJ, Parvataneni R, Molinaro A, Essock-Burns E, Bourne G, Parsa AT, Aghi MK, McDermott MW, Berger MS, Cha S, Chang SM, Nelson SJ (2012) Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR Imaging. Neuro Oncol 14:942–954. doi:10.1093/neuonc/nos128
Jain R, Gutierrez J, Narang J, Scarpace L, Schultz LR, Lemke N, Patel SC, Mikkelsen T, Rock JP (2011) In vivo correlation of tumor blood volume and permeability with histologic and molecular angiogenic markers in gliomas. AJNR Am J Neuroradiol 32:388–394. doi:10.3174/ajnr.A2280
Perry A, Schmidt RE (2006) Cancer therapy-associated CNS neuropathology: an update and review of the literature. Acta Neuropathol 111:197–212. doi:10.1007/s00401-005-0023-y
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Ellison DW, Figarella-Branger D, Perry A, Reifenberger G, von Deimling A (2016) World Health Organization classification of tumours of the central nervous system, 4th edn, chap 1. International Agency for Research on Cancer, pp 28–45
Boxerman JL, Prah DE, Paulson ES, Machan JT, Bedekar D, Schmainda KM (2012) The role of preload and leakage correction in gadolinium-based cerebral blood volume estimation determined by comparison with MION as a criterion standard. AJNR Am J Neuroradiol 33:1081–1087. doi:10.3174/ajnr.A2934
Paulson ES, Schmainda KM (2008) Comparison of dynamic susceptibility-weighted contrast-enhanced MR methods: recommendations for measuring relative cerebral blood volume in brain tumors. Radiology 249:601–613 doi:10.1148/radiol.2492071659
Prah MA, Stufflebeam SM, Paulson ES, Kalpathy-Cramer J, Gerstner ER, Batchelor TT, Barboriak DP, Rosen BR, Schmainda KM (2015) Repeatability of standardized and normalized relative CBV in patients with newly diagnosed glioblastoma. AJNR Am J Neuroradiol 36:1654–1661. doi:10.3174/ajnr.A4374
Bedekar D, Jensen T, Schmainda KM (2010) Standardization of relative cerebral blood volume (rCBV) image maps for ease of both inter- and intrapatient comparisons. Magn Reson Med 64:907–913. doi:10.1002/mrm.22445
Boxerman JL, Paulson ES, Prah MA, Schmainda KM (2013) The effect of pulse sequence parameters and contrast agent dose on percentage signal recovery in DSC-MRI: implications for clinical applications. AJNR Am J Neuroradiol 34:1364–1369. doi:10.3174/ajnr.A3477
Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) FSL. Neuroimage 62:782–790. doi:10.1016/j.neuroimage.2011.09.015
LaViolette PS, Rand SD, Ellingson BM, Raghavan M, Lew SM, Schmainda KM, Mueller W (2011) 3D visualization of subdural electrode shift as measured at craniotomy reopening. Epilepsy Res 94:102–109. doi:10.1016/j.eplepsyres.2011.01.011
Hu LS, Eschbacher JM, Heiserman JE, Dueck AC, Shapiro WR, Liu S, Karis JP, Smith KA, Coons SW, Nakaji P, Spetzler RF, Feuerstein BG, Debbins J, Baxter LC (2012) Reevaluating the imaging definition of tumor progression: perfusion MRI quantifies recurrent glioblastoma tumor fraction, pseudoprogression, and radiation necrosis to predict survival. Neuro Oncol 14:919–930. doi:10.1093/neuonc/nos112
Paulson ES, Prah DE, Schmainda KM (2016) Spiral perfusion imaging with consecutive echoes (SPICE) for the simultaneous mapping of DSC- and DCE-MRI parameters in brain tumor patients: theory and initial feasibility. Tomography 2:295–307. doi:10.18383/j.tom.2016.00217
Schmainda KM, Prah M, Baxter LC, Paulson ES, Maze S, Pipe J, Wang D, Debbins J, Hu LS (2016) Simultaneous measurement of DSC- and DCE-MRI parameters using dual-echo spiral with a standard dose of gadolinium in comparison to single-echo GRE-EPI methods in brain tumors. Proceedings of the Twenty-Third Meeting of the International Society for Magnetic Resonance in Medicine #487. Mira Smart Conferencing, Toronto, Ontario, Canada
Eidel O, Burth S, Neumann JO, Kieslich PJ, Sahm F, Jungk C, Kickingereder P, Bickelhaupt S, Mundiyanapurath S, Baumer P, Wick W, Schlemmer HP, Kiening K, Unterberg A, Bendszus M, Radbruch A (2017) Tumor infiltration in enhancing and non-enhancing parts of glioblastoma: a correlation with histopathology. PLoS ONE 12:e0169292. doi:10.1371/journal.pone.0169292
Chu HH, Choi SH, Ryoo I, Kim SC, Yeom JA, Shin H, Jung SC, Lee AL, Yoon TJ, Kim TM, Lee SH, Park CK, Kim JH, Sohn CH, Park SH, Kim IH (2013) Differentiation of true progression from pseudoprogression in glioblastoma treated with radiation therapy and concomitant temozolomide: comparison study of standard and high-b-value diffusion-weighted imaging. Radiology 269:831–840. doi:10.1148/radiol.13122024
Ye J, Bhagat SK, Li H, Luo X, Wang B, Liu L, Yang G (2016) Differentiation between recurrent gliomas and radiation necrosis using arterial spin labeling perfusion imaging. Exp Ther Med 11:2432–2436. doi:10.3892/etm.2016.3225
Larsen VA, Simonsen HJ, Law I, Larsson HB, Hansen AE (2013) Evaluation of dynamic contrast-enhanced T1-weighted perfusion MRI in the differentiation of tumor recurrence from radiation necrosis. Neuroradiology 55:361–369. doi:10.1007/s00234-012-1127-4
Kickingereder P, Sahm F, Radbruch A, Wick W, Heiland S, Deimling A, Bendszus M, Wiestler B (2015) IDH mutation status is associated with a distinct hypoxia/angiogenesis transcriptome signature which is non-invasively predictable with rCBV imaging in human glioma. Sci Rep 5:16238. doi:10.1038/srep16238
Xing Z, Yang X, She D, Lin Y, Zhang Y, Cao D (2017) Noninvasive assessment of IDH mutational status in World Health Organization grade II and III astrocytomas using DWI and DSC-PWI combined with conventional MR imaging. AJNR Am J Neuroradiol 38:1138–1144. doi:10.3174/ajnr.A5171
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. doi:10.1007/s00401-016-1545-1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ownership interest in Imaging Biometrics LLC (KMS). No conflict of interest to report (MAP, MMA, WMM, EJC, RGH, JMC).
Human Subjects Research
All procedures performed in this study were in accordance with the ethical standards of the Institution’s IRB and with the 1964 Helsinki declaration and its later amendments.
Animal Research
This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Prah, M.A., Al-Gizawiy, M.M., Mueller, W.M. et al. Spatial discrimination of glioblastoma and treatment effect with histologically-validated perfusion and diffusion magnetic resonance imaging metrics. J Neurooncol 136, 13–21 (2018). https://doi.org/10.1007/s11060-017-2617-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2617-3