Abstract
Purpose
The activity of the glymphatic system is impaired in animal models of Alzheimer’s disease (AD). We evaluated the activity of the human glymphatic system in cases of AD with a diffusion-based technique called diffusion tensor image analysis along the perivascular space (DTI-ALPS).
Materials and methods
Diffusion tensor images were acquired to calculate diffusivities in the x, y, and z axes of the plane of the lateral ventricle body in 31 patients. We evaluated the diffusivity along the perivascular spaces as well as projection fibers and association fibers separately, to acquire an index for diffusivity along the perivascular space (ALPS-index) and correlated them with the mini mental state examinations (MMSE) score.
Results
We found a significant negative correlation between diffusivity along the projection fibers and association fibers. We also observed a significant positive correlation between diffusivity along perivascular spaces shown as ALPS-index and the MMSE score, indicating lower water diffusivity along the perivascular space in relation to AD severity.
Conclusion
Activity of the glymphatic system may be evaluated with diffusion images. Lower diffusivity along the perivascular space on DTI-APLS seems to reflect impairment of the glymphatic system. This method may be useful for evaluating the activity of the glymphatic system.
Similar content being viewed by others
Introduction
The glymphatic system is a recently discovered waste drainage system in the brain that involves movement of the cerebrospinal fluid (CSF) along the perivascular space. This system promotes elimination of soluble proteins including amyloid-β (Aβ) and metabolites, and also facilitates the distribution of glucose, lipids, amino acids, and neuromodulators [1–3]. In this system, CSF and interstitial fluid (ISF) interchange by influx of CSF along the loose fibrous matrix of perivascular spaces [1]. CSF influx from the subarachnoid space into the deep periarterial space is driven by arterial pulsatile motion, slow motion of the vessels, respiratory motion, and CSF pressure. Subsequently, CSF is transported into the brain parenchyma via aquaporin-P4 (AQP4) water channels in astrocytic end-feet. CSF movement into the parenchyma drives convective ISF flux within the tissue toward the perivenous spaces surrounding the deep veins. ISF is then collected in the perivenous space. From the perivenous space, the fluid drains out of the brain toward the cervical lymphatic system [1, 4, 5]. These activities of the glymphatic system have been studied by intrathecally administered tracers in animal experiments [1]. However, this method is invasive in humans due to administration of tracers including gadolinium-based contrast medium (GBCM), and thus, a method has not been established for evaluation of the glymphatic system in humans.
The purpose of the current study was to assess the feasibility of a non-invasive method we coined “diffusion tensor image analysis along the perivascular space (DTI-ALPS)” for evaluating the activity of the glymphatic system in human brain by using diffusion images. In this method, we evaluated the motion of water molecules in the direction of the perivascular space by measuring diffusivity using the diffusion tensor method (Fig. 1). At the level of the lateral ventricle body, the medullary veins run perpendicular to the ventricular wall [6], and the perivascular space runs in the same direction as the medullary veins, which is the right-left direction (x-axis). On the plane of this area, projection fibers run in the head-foot direction, mainly adjacent to the lateral ventricle, and superior longitudinal fascicles (SLFs), representing association fibers in the current study, run in the anterior-posterior direction outside the projection fibers. Outside the SLFs, subcortical fibers run mainly in the right-left direction in subcortical areas. Consequently, in this area, the perivascular space runs perpendicular to the projection fibers and SLFs. This conformation of the perivascular space and major fibers in this area allows nearly independent analysis of the diffusivity along the direction of the perivascular space because major fiber tracts do not run parallel to the direction of the perivascular space. When there is histological change along the right-left direction (x-axis), it will equally affect both projection and association fibers. Thus, when such change is observed for both fiber bundles, it is probably safe to state that at least part of this change comes from the pathology involving the perivascular space, namely the glymphatic system.
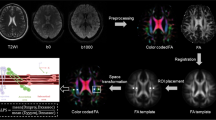
Concept for the diffusion tensor image analysis along the perivascular space (DTI-ALPS) method. a Roentgenogram of an injected coronal brain slice showing parenchymal vessels that run horizontally on the slice (white box) at the level of the lateral ventricle body. Reproduced with permission from [6]. b Axial SWI on the slice at the level of the lateral ventricle body indicates that parenchymal vessels run laterally (x-axis). c Superimposed color display of DTI on SWI (b) indicating the distribution of projection fibers (z-axis: blue), association fibers (y-axis: green), and the subcortical fibers (x-axis: red). Three ROIs are placed in the area with projection fibers (projection area), association fibers (association area) and subcortical fibers (subcortical area) to measure diffusivities of the three directions (x, y, z). d Schematic indicating the relationship between the direction of the perivascular space (gray cylinders) and the directions of the fibers. Note that the direction of the perivascular space is perpendicular to both projection and association fibers (color figure online)
Because the activity of the glymphatic system is reported to be impaired in AD according to observations in animal experiments [2], we analyzed the different degrees of glymphatic system activity by performing diffusion tensor studies of cases with Alzheimer’s disease (AD), mild cognitive impairment (MCI), and subjective cognitive impairment (SCI) with different degrees of severity.
Materials and methods
We studied 31 patients (14 males and 17 females; age range 51–89 years old; mean 75 years; median 76 years), 16 with AD, nine with MCI, and six with SCI [mini mental state examination (MMSE) range 12–30]. We obtained permission from the institutional review board at the institute where the imaging study was performed. Written informed consent for the imaging study was obtained from all patients or their families after the nature of the procedures had been fully explained. Clinical diagnoses of AD and MCI were based on the Diagnostic and Statistical Manual of Mental Disorders-IV criteria and examination of the cognitive status, and diagnosis of SCI was made according to the proposed Reisberg criteria for SCI [7]. All subjects were right-handed. MMSE was performed for all subjects.
Diffusion imaging was acquired by using a 3.0-T clinical scanner (Magnetom Verio, Siemens AG, Erlangen, Germany). DTI sets with b = 0, b = 1000, and b = 2000 s/mm2 (Echo planer, TR = 6600 ms, TE = 89 ms, MPG = 30 directions, FOV = 230 mm, matrix = 94 × 94, slice thickness = 3 mm) were acquired simultaneously in addition to conventional morphology images.
Diffusion metric images were generated by using dTV.II.13k+ software (Dept. Biomedical Information Sciences, Graduate School of Information Sciences, Hiroshima City University) [8]. The software creates computational images of the diffusion tensor including a color-coded fractional anisotropy (FA) map and diffusivity map, and in addition, has the capability to calculate diffusivity in the direction of the x-axis, y-axis, and z-axis on each image. Using this function, we performed analyses with the DTI-ALPS method, which is used to evaluate the diffusivity along the direction of the perivascular space compared with those of projection fibers and association fibers on a slice at the level of the lateral ventricle body (Fig. 1a). At that level, the direction of the perivascular space is perpendicular to the ventricle wall and is thus mostly in the right-left direction (x-axis) on the axial plane. The direction is also perpendicular to the direction of both the projection fibers (mostly in the z-axis) and the association fibers (mostly in the y-axis) (Fig. 1d). Thus, the diffusivity along the x-axis at regions with projection/association fibers will at least partly represent the diffusivity along the perivascular space. On a color-coded FA map of the plane at the level of the lateral ventricle body, we placed a 5-mm-diameter spherical region of interest (ROI) in the area of the projection fibers (blue on Fig. 1c), the area of the association fibers (green on Fig. 1c), and the area of the subcortical fibers (red on Fig. 1c) in the left hemisphere. For each area, we calculated the diffusivity in the directions of the x-axis, y-axis, and z-axis and correlated them with the MMSE score of the subjects to obtain a correlation coefficient and p value. We obtained measurements only in the left hemisphere in the current study, because all subjects were right-handed and the SLF is thick enough to place the ROI on the left side. We evaluated the statistical difference of the slope of the regression curves with analysis of co-variance (ANCOVA).
In addition, we calculated an index which we will call the ALPS-index in order to evaluate the activity of the glymphatic system in individual cases. This index is provided by the ratio of two sets of diffusivity value which are perpendicular to dominant fibers in the tissue, that is the ratio of mean of x-axis diffusivity in the area of projection fibers (Dxproj) and x-axis diffusivity in the area of association fibers (Dxassoc) to the mean of y-axis diffusivity in the area of projection fibers (Dyproj) and z-axis diffusivity in the area of association fibers (Dzaccoc) as follows.
In the area of projection fibers, the dominant fibers run in the direction of the z-axis, and both x-axis and y-axis are perpendicular to the dominant fibers. Similarly, in the area of association fibers, the dominant fibers run in the direction of the y-axis, and both x-axis and z-axis are perpendicular to the dominant fibers. The major difference for water molecule behavior between x-axis diffusivity in both areas (Dxproj and Dxassoc) and the diffusivity which is perpendicular to them (Dyproj and Dzassoc) would be the existence of the perivascular space. We also correlated this ALPS-index with the MMSE score. We made the above mentioned analyses separately for the datasets with b = 1000 and b = 2000 s/mm2.
Results
The correlations between MMSE and diffusivities for the three directions (x, y, z) of the three areas (projection, association, subcortical) are shown in Fig. 2 with their correlation coefficients and statistical significance (indicated as “*”). In the b = 1000 s/mm2 datasets in the projection area, we found a significant positive correlation (r = 0.40, p = 0.026) between the diffusivity along the perivascular space (x-axis) and the MMSE scores, whereas the diffusivity along the projection fibers (z-axis) showed a significant negative correlation (r = –0.62, p = 0.00021) with MMSE scores. Diffusivity in the anterior-posterior direction (y-axis) did not show a correlation with MMSE scores (r = –0.24, p = 0.20). Also in the b = 1000 s/mm2 datasets in the association area, we found a significant positive correlation (r = 0.50, p = 0.0042) between the diffusivity along the perivascular space (x-axis) and the MMSE scores, whereas the diffusivity along the association fibers (y-axis) showed a significant negative correlation (r = −0.55, p = 0.0013) with MMSE scores. Diffusivity in the head-feet direction (z-axis) did not show a correlation with MMSE scores (r = −0.10, p = 0.58). No statistically significant correlation was found between the diffusivity of the three directions and MMSE scores in the subcortical area.
Correlation between directional diffusivity and MMSE scores. Correlation between MMSE and diffusivities for the three directions of the three areas (projection a, d, association b, e, subcortical c, f) with the b = 1000 s/mm2 datasets (a, b, c) and the b = 2000 s/mm2 datasets (d, e, f). Diffusivity of the x-axis is plotted as red, y-axis as green, and z-axis as blue. Regression lines are also shown in the same colors with the plots, accompanied by values for the correlation coefficient. Statistically significant correlations are shown as asterisks. On the b = 1000 s/mm2 datasets, in the projection area (a), we found a significant positive correlation between the diffusivity along the perivascular space (x-axis) and the MMSE scores. In the association area (b), we found a significant positive correlation between the diffusivity along the perivascular space (x-axis) and the MMSE scores. On the other hand, there was a significant negative correlation between the diffusivity along the projection fibers (z-axis) in the projection area and the MMSE scores. There was also a significant negative correlation between the association fibers (y-axis) in the association area and the MMSE scores. These negative correlations may be explained by white matter degeneration in the projection or association fibers due to AD or MCI. On the b = 2000 s/mm2 measurements, we found no statistically significant correlations between the diffusivity and MMSE scores other than the diffusivity along the projection fibers (z-axis), which showed a significant negative correlation with MMSE scores in the projection area. Statistically significant differences (p < 0.001) for the regression line were identified with ANCOVA between the x-axis (red) and z-axis (blue) in the projection area on b = 1000 s/mm2 images (a) and between the x-axis (red) and y-axis (green) in the association area on b = 1000 s/mm2 images (b) (color figure online)
For the b = 2000 s/mm2 measurements, no statistically significant correlation was found between the diffusivity and MMSE scores other than the diffusivity along the projection fibers (z-axis), which showed a significant negative correlation (r = −0.48, p = 0.0067) with MMSE scores in the projection area. With ANCOVA, statistically significant differences were found in the slope of the regression line between the x-axis and z-axis in the projection area on b = 1000 s/mm2 images (p < 0.001) and between the x-axis and y-axis in the association area on b = 1000 s/mm2 images (p < 0.001).
On the evaluation of ALPS-index, there was a significant positive correlation (r = 0.46, p = 0.0084) between the ALPS-index and the MMSE scores on the b = 1000 s/mm2 datasets. While, on the b = 2000 s/mm2 measurements, there was not a statistically significant correlation between the ALPS-index and MMSE scores (r = 0.29, p = 0.109) (Fig. 3).
Correlation between ALPS-index and MMSE. Correlations between MMSE and ALPS-index, which is given by the following ratio, are shown (a b = 1000 s/mm2, b b = 2000 s/mm2). ALPS-index = mean (Dxproj, Dxassoc)/mean (Dyproj, Dzassoc). There was a significant positive correlation (r = 0.46, p = 0.0084) between the ALPS-index and the MMSE scores on the b = 1000 s/mm2 datasets (a). While, on the b = 2000 s/mm2 measurements (b), there was no statistically significant correlation between the ALPS-index and MMSE scores (r = 0.29, p = 0.109). Note that the cases with lower MMSE scores show lower ALPS-index, close to 1, while the ALPS-index has wide variability in the cases with high MMSE score
Discussion
The glymphatic system, which was first proposed 2012 by Nedergaard et al., is the waste clearance pathway system for CSF through the perivascular space and the interstitial space in the brain [1]. A recent report by Peng et al. indicated that glymphatic transport is suppressed in a mouse model of AD. They reported that Aβ circulates through glymphatic pathways. Oligomerization may limit the distribution of Aβ, and soluble Aβ oligomers, a more toxic form of Aβ, may also contribute to glymphatic dysfunction. Long-term exposure to Aβ in the subarachnoid CSF may suppress glymphatic transport prior to the presence of substantial Aβ deposits [9]. Apolipoprotein E4 (apoE4) is a major genetic risk factor for AD. However, how apoE4 influences AD onset and progression has yet to be ascertained. A recent experimental report showed that the glymphatic fluid transport system contributes to the delivery of choroid plexus/CSF-derived human apoE to neurons [10]. Perivascular localization of AQP4 facilitates the activity of the glymphatic system. Experimental mouse studies suggest that the AQP4-dependent glymphatic pathway is an important clearance system for driving the removal of soluble Aβ. In mice, Aβ is cleared along perivascular pathways, and Aβ clearance is reduced by 55–65% in AQP4 knockout mice compared with wild-type mice [1]. A recent report on postmortem human brain tissue showed that perivascular AQP4 localization is significantly associated with AD status independent of age [11].
Observation of the glymphatic system has been made with tracer studies in animal experiments [1–3]. Iliff et al. showed that CSF rapidly enters the brain along the cortical pial arteries following labeling of CSF with fluorescent tracers injected into the CSF of the cisterna magna. The dynamics of the glymphatic system were characterized for the first time in vivo using two-photon microscopy in mice [1]. In addition to fluorescence materials, intrathecal administration of GBCM was also used as a tracer in later animal studies. An experimental rat study with whole-brain imaging following intrathecal GBCM administration allowed identification of two key influx nodes at the pituitary and pineal gland recesses [2]. However, intrathecal administration of tracers in humans has not been performed except for several well-designed studies [12, 13] and an accident report [14]. GBCM administration only into CSF can cause T1 hyperintensity in the globus pallidus and dentate nucleus, strongly suggesting involvement of the glymphatic system in the phenomenon of gadolinium deposition in the globus pallidus or dentate nucleus [13]. Naganawa et al. observed enhancement of the perivascular space and CSF space on heavily T2-weighted FLAIR images 4 h after intravenous injection of GBCM [15, 16]; however, no method for tracing intravenously administered GBCM with MRI has been established. In addition, even if a tracer method to evaluate the glymphatic system in living humans is established, tracer studies of the glymphatic system require hours to follow the distribution of the tracer within the brain, and monitoring the activity of the glymphatic system in real time is not possible. Thus, monitoring methods other than tracer studies are needed to evaluate the glymphatic system. The DTI-ALPS method used in the current study uses diffusion images, which can be acquired within several minutes, and may have potential to monitor the status of the glymphatic system over time.
The DTI-ALPS method can be used to evaluate the diffusivity along the direction of the perivascular space compared with the direction of projection fibers and association fibers on a slice at the level of the lateral ventricle body. The medullary arteries and veins are the vessels of the brain parenchyma and accompany the perivascular space, which is the major draining pathway of the glymphatic system. It is still difficult to visualize fine medullary arteries on MR images, however, fine medullary veins can be visualized with use of 3-T scanners and high resolution imaging protocols such as susceptibility-weighted imaging (SWI) [17]. Prior to the current evaluation, we examined SWIs and color-coded FA images of normal brains and looked for the area in which conformation of the perivascular space, represented by medullary veins on SWI, and the major white matter tracts were perpendicular to each other. We found that the perivascular space runs in the right-left direction (x-axis), projection fibers run in the head-foot (z-axis) direction, and association fibers run in the anterior-posterior (y-axis) direction outside the projection fibers at the level of the lateral ventricle body.
Our results of the current study indicated a significant negative correlation between MMSE scores and the diffusivity along the projection fibers and association fibers. This result can be explained by white matter degeneration in the projection or association fibers due to AD or MCI as shown in a previous report [18]. In addition, an atlas-based analysis of DTI to survey 30 major cerebral white matter tracts in AD cases indicated that association tracts have increases in mean, axial, and radial diffusivity, and projection tracts have increases in axial and radial diffusivity, observations that are interpreted as decreased tissue density in these tracts in AD cases [19]. In contrast, our current study showed a significant positive correlation between the diffusivity along the perivascular space and the MMSE score, indicating impaired water diffusivity in the direction of the perivascular space in relation to AD severity in areas with projection or association fiber dominance. Our results are consistent with the impaired activity of the glymphatic system in AD suggested by experiments with rats [2]. We made this evaluation with two different b values including b = 1000 s/mm2 as the standard b value and b = 2000 s/mm2 as the higher b value. In order to assure better signal to noise ratio, we did not selected a very high b value such as b = 3000 s/mm2. The observations above, such as positive correlation between ALPS-index and MMSE, were more obvious in the measurement with b = 1000 s/mm2 than with b = 2000 s/mm2. Since the influence from water molecules with higher motivity becomes dominant when the b value is lower, higher diffusivity or motivity of water molecules within the perivascular space could have a larger influence with a rather lower b value (b = 1000 s/mm2) measurement. Another possible reason for the difference between b = 1000 and b = 2000 s/mm2 is the higher signal to noise ratio which is acquired in the measurement with b = 1000 s/mm2.
We calculated the ALPS-index in order to evaluate the activity of the glymphatic system in individual cases. In this index, we hypothesized that the ratio of x-axis diffusivity in the projection fibers and association fibers area (Dxproj and Dxassoc) to the diffusivity which is perpendicular to them (Dyproj and Dzassoc) would express the influence of the water diffusion along the perivascular space which will reflect activity of the glymphatic system in the individual cases. When the ratio is close to 1, it means that the influence of the water diffusion along the perivascular space is minimal, and a larger ratio will represent larger water diffusivity along the perivascular space. In our results, there was a significant positive correlation between the ALPS-index and the MMSE scores on the b = 1000 s/mm2 datasets. This result would indicate that the ALPS-index can be used for evaluating the activity of the glymphatic system in individual cases. The result also indicated that the cases with a lower MMSE score were only found with a lower ALPS-index. This may indicate that the cases with severe AD have an impaired glymphatic system, almost without exception. On the other hand, the ALPS-index has wide variability in the cases with rather high MMSE score. There may be other unknown factors which impair the glymphatic system in some cases with mild AD, or in MCI and SCI cases.
Our current study has several limitations. At the moment, our software can calculate the diffusivity only in x, y and z axes, which are obtained as the components of diffusion tensors; Dxx, Dyy, and Dzz. Therefore, the area outside the lateral ventricle along the plane of the lateral ventricle body is the only place where we can independently evaluate the diffusivity along the direction of the perivascular space. In areas in which the perivascular space does not run in the direction of the x, y, or z axes or areas in which the perivascular space and the direction of the predominant fiber tract are parallel, isolated evaluation of the diffusivity along the perivascular space is impossible. Another limitation is that the ROI was placed manually, which may be a subjective factor of our measurement. However, we tried to place ROI as objectively as possible. The small number of subjects and the fact that the study was performed at a single institute are other limitations. In addition, we did not take any other physiological statuses into account, including perfusion or pulsatile motion of the brain. However, we performed diffusion imaging with b = 1000 and b = 1000 s/mm2, because this rather high motion gradient is expected to cancel out the rather macroscopic physiological status.
In conclusion, our results using the DTI-ALPS method demonstrated a significant positive correlation between the diffusivity along the perivascular space and the MMSE score, indicating impaired water diffusivity related to AD, MCI, or SCI severity. Thus, lower diffusivity along the perivascular space or lower ALPS-index seems to reflect impairment of the glymphatic system. The DTI-APLS method can be used for evaluating the activity of the glymphatic system, and the ALPS-index may be applied for evaluating conditions that affect the activity of the glymphatic system of individual cases.
References
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra11.
Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123(3):1299–309.
Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: a beginner’s guide. Neurochem Res. 2015;40(12):2583–99.
Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004;1(1):2.
Murtha LA, Yang Q, Parsons MW, Levi CR, Beard DJ, Spratt NJ, et al. Cerebrospinal fluid is drained primarily via the spinal canal and olfactory route in young and aged spontaneously hypertensive rats. Fluids Barriers CNS. 2014;11:12.
Okudera T, Huang YP, Fukusumi A, Nakamura Y, Hatazawa J, Uemura K. Micro-angiographical studies of the medullary venous system of the cerebral hemisphere. Neuropathology. 1999;19(1):93–111.
Reisberg B, Prichep L, Mosconi L, John ER, Glodzik-Sobanska L, Boksay I, et al. The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimers Dement. 2008;4(1 Suppl 1):S98–108.
Masutani Y, Aoki S, Abe O, Hayashi N, Otomo K. MR diffusion tensor imaging: recent advance and new techniques for diffusion tensor visualization. Eur J Radiol. 2003;46(1):53–66.
Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2016;93:215–25.
Achariyar TM, Li B, Peng W, Verghese PB, Shi Y, McConnell E, et al. Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol Neurodegener. 2016;11(1):74.
Zeppenfeld DM, Simon M, Haswell JD, D’Abreo D, Murchison C, Quinn JF, et al. Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains. JAMA Neurol. 2016;74(1):91–9.
Tali ET, Ercan N, Krumina G, Rudwan M, Mironov A, Zeng QY, et al. Intrathecal gadolinium (gadopentetate dimeglumine) enhanced magnetic resonance myelography and cisternography: results of a multicenter study. Invest Radiol. 2002;37(3):152–9.
Oner AY, Barutcu B, Aykol S, Tali ET. Intrathecal contrast-enhanced magnetic resonance imaging-related brain signal changes: residual gadolinium deposition? Invest Radiol 2016. doi:10.1097/RLI.0000000000000327
Samardzic D, Thamburaj K. Magnetic resonance characteristics and susceptibility weighted imaging of the brain in gadolinium encephalopathy. J Neuroimaging. 2015;25(1):136–9.
Naganawa S, Nakane T, Kawai H, Taoka T. Gd-based contrast enhancement of the perivascular spaces in the basal ganglia. Magn Reson Med Sci. 2016;16(1):61–5.
Naganawa S, Suzuki K, Yamazaki M, Sakurai Y. Serial scans in healthy volunteers following intravenous administration of gadoteridol: time course of contrast enhancement in various cranial fluid spaces. Magn Reson Med Sci. 2014;13(1):7–13.
Taoka T, Fukusumi A, Miyasaka T, Kawai H, Nakane T, Kichikawa K, et al. Structure of the medullary veins of the cerebral hemisphere and related disorders. Radiographics. 2017;37(1):281–97.
Fellgiebel A, Yakushev I. Diffusion tensor imaging of the hippocampus in MCI and early Alzheimer’s disease. J Alzheimers Dis. 2011;26(Suppl 3):257–62.
Huang H, Fan X, Weiner M, Martin-Cook K, Xiao G, Davis J, et al. Distinctive disruption patterns of white matter tracts in Alzheimer’s disease with full diffusion tensor characterization. Neurobiol Aging. 2012;33(9):2029–45.
Acknowledgements
Funding was provided by Grant-in-Aid for Scientific Research (Grant Nos. 16K10312 and 26461834).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
All applicable institutional and/or national guidelines for care were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Taoka, T., Masutani, Y., Kawai, H. et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol 35, 172–178 (2017). https://doi.org/10.1007/s11604-017-0617-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-017-0617-z