Abstract
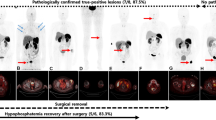
Diagnosing tumor-induced osteomalacia is often challenging because conventional imaging modalities may fail to locate the responsible tumor. This report describes the ability of 68Ga-DOTATOC PET/CT to successfully distinguish between the responsible phosphaturic mesenchymal tumor and concurrent lymphoma lesions. A 52-year-old man with bone pain for several years was diagnosed with a vitamin D-resistant hypophosphatemic osteomalacia. Whole body 18F-FDG PET/CT revealed multiple enlarged hypermetabolic lymph nodes in his bilateral cervical, axillary, mediastinal, abdominal, pelvic, and inguinal regions. Core needle biopsy of the right cervical lymph node confirmed the diagnosis of follicular lymphoma. However, lymphoma was not considered the cause of osteomalacia. 68Ga-DOTATOC PET/CT before chemotherapy showed a small nodule with intensely increased uptake in the right inguinal region, which was distinguished from the other enlarged lymph nodes. The nodule was surgically removed and histopathologically consistent with phosphaturic mesenchymal tumor. After surgery, the patient’s serum phosphorus and alkaline phosphatase levels normalized without nutritional supplement.





Similar content being viewed by others
References
Dupond JL, Mahammedi H, Prie D, Collin F, Gil H, Blagosklonov O, et al. Oncogenic osteomalacia: diagnostic importance of fibroblast growth factor 23 and F-18 fluorodeoxyglucose PET/CT scan for the diagnosis and follow-up in one case. Bone. 2005;36:375–8.
Paquet M, Gauthe M, Zhang Yin J, Nataf V, Belissant O, Orcel P, et al. Diagnostic performance and impact on patient management of (68)Ga-DOTA-TOC PET/CT for detecting osteomalacia-associated tumours. Eur J Nucl Med Mol Imaging. 2018;45:1710–20.
Yu WJ, He JW, Fu WZ, Wang C, Zhang ZL. Reports of 17 Chinese patients with tumor-induced osteomalacia. J Bone Miner Metab. 2017;35:298–307.
Jadhav S, Kasaliwal R, Lele V, Rangarajan V, Chandra P, Shah H, et al. Functional imaging in primary tumour-induced osteomalacia: relative performance of FDG PET/CT vs somatostatin receptor-based functional scans: a series of nine patients. Clin Endocrinol. 2014;81:31–7.
Clifton-Bligh RJ, Hofman MS, Duncan E, Sim Ie W, Darnell D, Clarkson A, et al. Improving diagnosis of tumor-induced osteomalacia with Gallium-68 DOTATATE PET/CT. J Clin Endocrinol Metab. 2013;98:687–94.
King AL, Sica DA, Miller G, Pierpaoli S. Severe hypophosphatemia in a general hospital population. South Med J. 1987;80:831–5.
Gaasbeek A, Meinders AE. Hypophosphatemia: an update on its etiology and treatment. Am J Med. 2005;118:1094–101.
Weidner N, Santa Cruz D. Phosphaturic mesenchymal tumors. A polymorphous group causing osteomalacia or rickets. Cancer. 1987;59:1442–54.
Huang X, Jiang Y, Xia W. FGF23 and phosphate wasting disorders. Bone Res. 2013;1:120–32.
Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev. 2012;92:131–55.
Shiba E, Matsuyama A, Shibuya R, Yabuki K, Harada H, Nakamoto M, et al. Immunohistochemical and molecular detection of the expression of FGF23 in phosphaturic mesenchymal tumors including the non-phosphaturic variant. Diagn Pathol. 2016;11:26.
Imel EA, Peacock M, Pitukcheewanont P, Heller HJ, Ward LM, Shulman D, et al. Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J Clin Endocrinol Metab. 2006;91:2055–61.
Folpe AL, Fanburg-Smith JC, Billings SD, Bisceglia M, Bertoni F, Cho JY, et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004;28:1–30.
Zuo QY, Wang H, Li W, Niu XH, Huang YH, Chen J, et al. Treatment and outcomes of tumor-induced osteomalacia associated with phosphaturic mesenchymal tumors: retrospective review of 12 patients. BMC Musculoskelet Disord. 2017;18:403.
Chong WH, Andreopoulou P, Chen CC, Reynolds J, Guthrie L, Kelly M, et al. Tumor localization and biochemical response to cure in tumor-induced osteomalacia. J Bone Miner Res. 2013;28:1386–98.
Jagtap VS, Sarathi V, Lila AR, Malhotra G, Sankhe SS, Bandgar T, et al. Tumor-induced osteomalacia: a single center experience. Endocr Pract. 2011;17:177–84.
El-Maouche D, Sadowski SM, Papadakis GZ, Guthrie L, Cottle-Delisle C, Merkel R, et al. (68)Ga-DOTATATE for tumor localization in tumor-induced osteomalacia. J Clin Endocrinol Metab. 2016;101:3575–81.
Honda R, Kawabata Y, Ito S, Kikuchi F. Phosphaturic mesenchymal tumor, mixed connective tissue type, non-phosphaturic variant: report of a case and review of 32 cases from the Japanese published work. J Dermatol. 2014;41:845–9.
Agaimy A, Michal M, Chiosea S, Petersson F, Hadravsky L, Kristiansen G, et al. Phosphaturic mesenchymal tumors: clinicopathologic, immunohistochemical and molecular analysis of 22 cases expanding their morphologic and immunophenotypic spectrum. Am J Surg Pathol. 2017;41:1371–80.
Dadoniene J, Miglinas M, Miltiniene D, Vajauskas D, Seinin D, Butenas P, et al. Tumour-induced osteomalacia: a literature review and a case report. World Journal of Surgical Oncology. 2016;14:4.
Hautmann AH, Hautmann MG, Kolbl O, Herr W, Fleck M. Tumor-induced osteomalacia: an up-to-date review. Curr Rheumatol Rep. 2015;17:512.
Jiang Y, Xia WB, Xing XP, Silva BC, Li M, Wang O, et al. Tumor-induced osteomalacia: an important cause of adult-onset hypophosphatemic osteomalacia in China: report of 39 cases and review of the literature. J Bone Miner Res. 2012;27:1967–75.
Deep NL, Cain RB, McCullough AE, Hoxworth JM, Lal D. Sinonasal phosphaturic mesenchymal tumor: case report and systematic review. Allergy Rhinol (Providence). 2014;5:162–7.
Shi Z, Deng Y, Li X, Li Y, Cao D, Coossa VS. CT and MR imaging features in phosphaturic mesenchymal tumor-mixed connective tissue: a case report. Oncol Lett. 2018;15:4970–8.
Seo HJ, Choi YJ, Kim HJ, Jeong YH, Cho A, Lee JH, et al. Using (18)F-FDG PET/CT to detect an occult mesenchymal tumor causing oncogenic osteomalacia. Nucl Med Mol Imaging. 2011;45:233–7.
Hautmann AH, Schroeder J, Wild P, Hautmann MG, Huber E, Hoffstetter P, et al. Tumor-induced osteomalacia: increased level of FGF-23 in a patient with a phosphaturic mesenchymal tumor at the tibia expressing periostin. Case Reports in Endocrinology. 2014;2014:7.
Malhotra G, Agrawal A, Jambhekar NA, Sarathi V, Jagtap V, Agarwal MG, et al. The search for primary tumor in a patient with oncogenic osteomalacia: F-18 FDG PET resolves the conundrum. Clin Nucl Med. 2010;35:896–8.
Houang M, Clarkson A, Sioson L, Elston MS, Clifton-Bligh RJ, Dray M, et al. Phosphaturic mesenchymal tumors show positive staining for somatostatin receptor 2A (SSTR2A). Hum Pathol. 2013;44:2711–8.
Johnbeck CB, Knigge U, Kjaer A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol. 2014;10:2259–77.
Miederer M, Seidl S, Buck A, Scheidhauer K, Wester HJ, Schwaiger M, et al. Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur J Nucl Med Mol Imaging. 2009;36:48–52.
Agrawal K, Bhadada S, Mittal BR, Shukla J, Sood A, Bhattacharya A, et al. Comparison of 18F-FDG and 68Ga DOTATATE PET/CT in localization of tumor causing oncogenic osteomalacia. Clin Nucl Med. 2015;40:e6–e10.
Singh D, Chopra A, Ravina M, Kongara S, Bhatia E, Kumar N, et al. Oncogenic osteomalacia: role of Ga-68 DOTANOC PET/CT scan in identifying the culprit lesion and its management. Br J Radiol. 2017;90:20160811.
Hofman MS, Lau WFE, Hicks RJ. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics. 2015;35:500–16.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sejin Ha, Sujin Park, Hyunji Kim, Heounjeong Go, Seung Hun Lee, Ji Yoon Choi, Jung Yong Hong, and Jin-Sook Ryu declare that they have no conflicts of interest.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the respective institutional research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
The study protocol was approved by the institutional review board of our institute, which waived the requirement for informed consent due to the retrospective nature of the study (IRB no. 2018-1391-0001).
Rights and permissions
About this article
Cite this article
Ha, S., Park, S., Kim, H. et al. Successful Localization Using 68Ga-DOTATOC PET/CT of a Phosphaturic Mesenchymal Tumor Causing Osteomalacia in a Patient with Concurrent Follicular Lymphoma. Nucl Med Mol Imaging 52, 462–467 (2018). https://doi.org/10.1007/s13139-018-0546-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-018-0546-5