Abstract
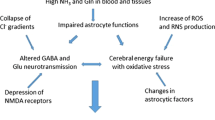
Hepatic encephalopathy (HE) is an important cause of morbidity and mortality in patients with severe liver disease. Although the molecular basis for the neurological disorder in HE remains elusive, elevated ammonia and its chief metabolite glutamine are believed to be important factors responsible for altered cerebral functions, including multiple neurotransmitter system(s) failure, altered bioenergetics, and more recently oxidative stress. Accumulated evidence suggests that direct interference of ammonia at several points in cerebral energy metabolism, including glycolysis, TCA cycle, and the electron transport chain, could lead to energy depletion. Additionally, recent studies from our laboratory have invoked the possibility that ammonia and glutamine may induce the mitochondrial permeability transition in astrocytes, a process capable of causing mitochondrial dysfunction. Altered mitochondrial metabolism appears to be an important mechanism responsible for the cerebral abnormalities associated with HE and other hyperammonemic states.
Similar content being viewed by others
REFERENCES
Albrecht, J., Hilgier, W., and Rafalowska, U. (1990). Activation of arginine metabolism to glutamate in rat brain synaptosomes in thioacetamide-induced hepatic encephalopathy: An adaptive response? J. Neurosci. Res. 25:125–130.
Astore, D., and Boicelli, C.A. (2000). Hyperammonemia and chronic hepatic encephalopathy: An in vivo PMRS study of rat brain. MAGMA 10:160–166.
Audet, R., and Butterworth, R.F. (1998). Porta-caval anastomosis results in more severe neurological impairment and more widespread reductions in cerebral metabolism in old versus young adult rats: Implications for post-TIPS encephalopathy. Metab. Brain Dis. 13:69–78.
Bai, G., Murthy, Ch.R.K., and Norenberg, M.D. (2000). Ammonia induces mitochondrial permeability transition in cultured astrocytes. Soc. Neurosci. Abstr. 26:1893.
Baraona, E.A., Salinas, A., Navia, E., and Orego, H. (1965). Alterations of ammonia metabolism in cerebral cortex of rats with hepatic damage induced by carbon tetrachloride. Clin. Sci. Lond. 28:201–208.
Benjamin, A.M., and Quastel, J.H. (1972). Locations of amino acids in brain cortex slices from the rat. Tetrodotoxinsensitive release of amino acids. Biochem. J. 128:631–646.
Bernardi, P. (1996). The permeability transition pore. Control points of a cyclosporin A sensitive mitochondrial channel involved in cell death. Biochim. Biophys. Acta 1275:5–9.
Bernardi, P., Colonna, R., Costantini, P., Eriksson, O., Fontaine, E., Ichas, F., Massari, S., Nicolli, A., Petronilli, V., and Scorrano, L. (1998). The mitochondrial permeability transition. Biofactors 8:273–281.
Bessman, S.P., and Bessman, A.N. (1955). The cerebral and peripheral uptake of ammonia in liver disease with a hypothesis for mechanism of hepatic coma. J. Clin. Invest. 34:622–628.
Bessman, S.P., and Paul, N. (1976). The Krebs's cycle depletion theory of hepatic coma. In (S. Grisolia, R. Begone, and F. Meyer, eds.) Urea Cycle, Wiley, New York, pp. 83–89.
Bosman, D.K., Deutz, N.E., deGraff, A.A., van Eijk, H.M., Bovee, W.M., Maas, M.A., Jorning, G.G., and Chamuleau, R.A. (1990). Changes in brain metabolism during hyperammonemia and acute liver failure: Results of a comparative 1H-NMR spectroscopy and biochemical investigation. Hepatology 12:281–290.
Bradford, H.F., and Ward, D.K. (1976). On glutaminase activity in mammalian synaptosomes. Brain Res. 110:115–125.
Butterworth, R.F. (1991). Pathophysiology of hepatic encephalopathy: The ammonia hypothesis revisited. In (F. Bengtsson, B. Jeppsson, T. Aamdal, and H. Vistrup, eds.) Progress in Hepatic Encephalopathy and Metabolic Nitrogen Exchange, CRC Press, Boca Raton, pp. 9–24.
Buzanska, L., Zablocka, B., Dybel, A., Domanska-Janik, K., and Albrecht, J. (2000). Delayed induction of apoptosis by ammonia in C6 glioma cells. Neurochem. Int. 37:287–297.
Cataldo, A.M., and Broadwell, R.D. (1986). Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. II. Choroid plexus and ependymal epithelia, endothelia and pericytes. J. Neurocytol. 15:511–524.
Cooper, A.J.L., and Plum, F. (1987). Biochemistry and physiology of brain ammonia. Physiol. Rev. 67:440–519.
Crompton, M., Cost, A., and Hayat, L. (1987). Evidence for the presence of a reversible Ca2+-dependent pore activated by oxidative stress in heart mitochondria. Biochem. J. 245:915–918.
Cruz, N.F., and Dienel, G.A. (1994). Brain glucose levels in porta-caval-shunted rats with chronic, moderate hyperammonemia: Implications for determination of local cerebral glucose utilization. J. Cereb. Blood Flow Metab. 14:113–124.
Cruz, N.F., and Duffy, T.E. (1983). Local cerebral glucose metabolism in rats with chronic porta-caval shunts. J. Cereb. Blood Flow Metab. 3:311–320.
Dejong, C.H., Kampmann, M.T., Deutz, N.E., and Soeters, P.B. (1992). Cerebral cortex ammonia and glutamine metabolism during liver insufficiency-induced hyperammonemia in the rat. J. Neurochem. 59:1071–1079.
Dombro, R.S., Hutson, D.K., and Norenberg, M.D. (1993). Action of ammonia on astrocyte glycogen and glycogenolysis. Mol. Chem. Neuropathol. 19:259–268.
Faff-Michalak, L., and Albrecht, J. (1991). Aspartate aminotransferase, malate dehydrogenase and pyruvate dehydrogenase activities in rat cerebral synaptic and nonsynaptic mitochondria: Effects of in vitro treatment with ammonia, hyperammonemia and hepatic encephalopathy. Metab. Brain Dis. 6:187–197.
Fitzpatrik, S.M., Cooper, A.J., and Duffy, T.E. (1983). Effect of ammonia and β-methylene aspartate on the oxidation of glucose and pyruvate by neurons and astrocytes in primary culture. J. Neurochem. 41:1370–1383.
Giguère, J.F., Besnard, A.M., Lavoie, J., Pomier Layrargues, G., and Butterworth, R.F. (1989). Activities of glutamate-related enzymes in autopsied brain tissue from cirrhotic patients with hepatic encephalopathy. In (R.F. Butterworth and G. Pomier Layrargues, eds.) Hepatic Encephalopathy: Pathophysiology and Treatment, Humana Press, Clifton, NJ, pp. 435–446.
Gregorios, J.B., Mozes, L.W., and Norenberg, M.D. (1985). Morphologic effects of ammonia on primary astrocyte cultures. II. Electron microscopic studies. J. Neuropathol. Exp. Neurol. 44:404–414.
Guerrini, V.H. (1994). Effect of antioxidants on ammonia induced CNS-renal pathobiology in sheep. Free Radic. Res. 21:35–43.
Gunter, T.E., and Pfeiffer, D.R. (1990). Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 258:C755–C786.
Haghighat, N., and McCandless, D.R. (1997). Effect of ammonium chloride on energy metabolism of astrocytes and C-6 glioma cells in vitro. Metab. Brain Dis. 12:287–289.
Hamberger, A., Lindorth, B., and Nystrom, B. (1982). Regulation of glutamate biosynthesis and release in vitro by low levels of ammonium ions. Brain Res. 237:339–350.
Hawkins, R.A., and Jessy, J. (1991). Hyperammonemia does not impair brain function in the absence of net glutamine synthesis. Biochem. J. 277:697–703.
Hawkins, R.A., and Mans, A.M. (1989). Brain energy metabolism in hepatic encephalopathy. In (R.F. Butterworth and G. Pomier Layrargues, eds.) Hepatic encephalopathy: Pathophysiology and Treatment. Humana Press, Clifton, NJ, pp. 159–176.
Hawkins, R.A., Mans, A.M., and Biebuyck, R.J.F. (1987). Changes in brain metabolism in hepatic encephalopathy. Neurochem. Pathol. 6:35–66.
Hawkins, R.A., Miller, A.L., Nielsen, R.C., and Veech, R.L. (1973). The acute action of ammonia on rat brain metabolism in vivo. Biochem. J. 134:1001–1008.
Hazell, A.S., and Butterworth, R.F. (1999). Hepatic encephalopathy: An update of pathophysiologic mechanisms. Soc. Exptl. Biol. and Med. 222:99–112.
Hazell, A.S., and Norenberg, M.D. (1998). Ammonia and manganese increase arginine uptake in cultured astrocytes. Neurochem. Res. 23:869–873.
Heales, S.J.R., Bolanos, J.P., Stewart, V.C., Brookes, P.S., and Clark, J.B. (1999). Nitric oxide, mitochondria and neurological disease. Biochim. Biophys. Acta 1410:215–228.
Hindfelt, B. (1975). On mechanisms in hyperammonemic coma with particular reference to hepatic encephalopathy. Ann. N.Y. Acad. Sci. 252:116–123.
Hindfelt, B. (1984). Effect of sustained portal-systemic shunting on glycolytic and citric acid cycle intermediates in the rat brain. Euro. J. Clin. Invest. 14:334–338.
Hindfelt, B., Plum, F., and Duffy, T.E. (1977). Effect of acute ammonia intoxication on cerebral metabolism in rats with porta-caval shunts. J. Clin. Invest. 59:386–396.
Hindfelt, B., and Seisjõ, B.K. (1971). Cerebral effects of acute ammonia intoxication. II. The effect upon energy metabolism. Scard. J. Clin. Lab. Invest. 127:1033–1036.
Hirsch, T., Marchetti, P., Susin, S.A., Dallaporta, B., Zamzami, N., Marzo, I., Geuskens, M., and Kroemer, G. (1997). The apoptosis-necrosis paradox. Apoptogenic proteases activated after mitochondrial permeability transition determine the mode of cell death. Oncogene 15:1573–1581.
Ide, T., Tsutsui, H., Kinugawa, S., Utsuimi, H., Kang, D., Hattori, N., Uchida, K., Arimura, Ki., Egashira, K., and Takeshita, A. (1999). Mitochondrial electron transport chain complex I is a potential source of free radicals in the failing myocardium. Circ. Res. 85:357–363.
Katanuma, N., Okada, M., and Nishii, Y. (1966). Regulation of the urea cycle and TCA cycle by ammonia. Adv. Enzyme Regul. 4:317–335.
Kerr, P.M., Suleiman, M.S., and Halestrap, A.P. (1999). Reversal of permeability transition during recovery of hearts from ischemia and its enhancement by pyruvate. Am. J. Physiol. 276:H496–502.
Kosenko, E., Felipo, V., Minana, M.D., Grau, E., and Grisolia, S. (1991). Ammonium ingestion prevents depletion of hepatic energy metabolites induced by acute ammonium intoxication. Arch. Biochem. Biophys. 290:484–488.
Kosenko, E., Felipo, V., Montoliu, C., Grisolia, S., and Kaminsky, Y. (1996). Effects of acute hyperammonemia in vivo on oxidative metabolism in nonsynaptic rat brain mitochondria. Metab. Brain Dis. 12:69–82.
Kosenko, E., Kaminsky, Y.G., Felipo, V., Minana, M.D., and Grisolia, S. (1993). Chronic hyperammonemia prevents changes in brain energy and ammonia metabolites induced by acute ammonium intoxication. Biochim. Biophys. Acta 1180:321–326.
Kosenko, E., Kaminsky, Y., Gran, E., Minana, M.D., Grisolia, S., and Felipo, V. (1994). Brain ATP depletion induced by acute ammonia intoxication in rats is mediated by activation of NMDA receptors and NaC-KCATPase. J. Neurochem. 63:2172–2178.
Kosenko, E., Kaminsky, Y., Kaminsky, A., Valencia, M., Lee, L., Hermenegildo, C., and Felipo, V. (1997). Superoxide production and antioxidant enzymes in ammonia intoxication in rats. Free Rad. Res. 27:636–644.
Kosenko, E., Kaminski, Y., Lopata, O., Muravyov, N., and Felipo, V. (1999). Blocking NMDA receptors prevents the oxidative stress induced by acute ammonia intoxication. Free Radic. Biol. Med. 26:1369–1374.
Kosenko, E., Kaminsky, Y., Lopata, O., Muravyov, N., Kaminsky, A., Hermengildo, C., and Felipo, V. (1998). Nitro-arginine, an inhibitor of nitric oxide synthase, prevents changes in superoxide radical and antioxidant enzymes induced by ammonia intoxication. Metab. Brain Dis. 13:29–41.
Kroemer, G., Dallaporta, B., and Resche-Rigon, M. (1998). The mitochondrial death/life regulator in apoptosis and necrosis. Ann. Rev. Physiol. 6:619–642.
Lai, J.C.K., and Cooper, A.J.L. (1986). Brain α-ketoglutarate dehydrogenase complex: Kinetic properties, regional distribution and effects of inhibitors. J. Neurochem. 47:1376–1386.
Lai, J.C.K., Murthy, Ch.R.K., Cooper, A.J.L., Hertz, E., and Hertz, L. (1989). Differential effects of ammonia and β-methyleneaspartate on the metabolism of glutamate and related amino acids by astrocytes and neurons in primary culture. Neurochem. Res. 14:377–389.
Lavoie, J., Giguere, J.F., Layrargues, G.P., and Butterworth, R.F. (1987). Amino acid changes in autopsied brain tissue from cirrhotic patients with hepatic encephalopathy. J. Neurochem. 49:692–697.
Lin, S., and Raabe, W. (1985). Ammonia intoxication: Effects on cerebral cortex and spinal cord. J. Neurochem. 44:1252–1258.
Lockwood, A.H., Ginsberg, M.D., Buttler, C.M., and Gutierrez, M.T. (1982). Selective effects of ammonia on regional brain glucose metabolism. Ann. Neurol. 12:114.
Matsuoka, M., Igisu, H., Kohriyama, K., and Inoue, N. (1990). Effect of ammonia on brain energy metabolites: dose-dependent alterations. J. Neurochem. 55:325–334.
McCandless, D.W., and Schenker, S. (1981). Effect of acute ammonia intoxication on energy stores in the cerebral reticular activation system. Exp. Brain Res. 44:325–330.
McKhann, G.M., and Tower, D.B. (1961). Ammonia toxicity and cerebral oxidative metabolism. Am. J. Physiol. 200:420–424.
Minamikawa, T., Williams, D.A., Bowser, D.N., and Nagley, P. (1999). Mitochondrial permeability transition and swelling can occur reversibly without inducing cell death in intact human cells. Exp. Cell Res. 246:26–37.
Mousseau, D.D., and Butterworth, R.F. (1994). Current theories on the pathogenesis of hepatic encephalopathy. Proc. Soc. Exp. Biol. Med. 206:329–344.
Muntz, J.A., and Hurwitz, J. (1951). The effect of ammonium ions upon isolated reactions of glycolytic scheme. Arch. Biochem. Biophys. 32:137–149.
Murphy, M.G., Jollimore, C., Crocker, J.F.S., and Her, H. (1992). Beta-oxidation of [1-14C]-palmitic acid by mouse astrocytes in primary culture. Effects of agents implicated in encephalopathy of Reye's syndrome. J. Neurosci. Res. 33:445–454.
Murthy, Ch.R.K., and Hertz, L. (1988). Pyruvate decarboxylation in astrocytes and neurons in primary cultures in the presence and absence of ammonia. Neurochem. Res. 13:57–61.
Murthy, Ch.R.K., Liu, H., and Norenberg, M.D. (2000). Ammonia-induced free radical production in primary cultures of astrocytes. J. Neurochem. 74 (Suppl.): S 81 A
Norenberg, M.D. (1981). The astrocytes in liver disease. Adv. Cell. Neurobiol. 2:303–351.
Norenberg, M.D. (1987). Role of astrocytes in hepatic encephalopathy. Neurochem. Pathol. 6:13–33.
Norenberg, M.D., Huo, Z., Neary, J.T., and Roig-Cantesano, A. (1997). Glial glutamate transporter in hyperammonemia and hepatic encephalopathy: Relation to energy metabolism and glutamatergic neurotransmitter function. Glia 21:124–133.
Norenberg, M.D., and Itzhak, Y. (1995). Acute liver failure and hyperammonemia increase neuronal nitric oxide synthase in mouse brain. Soc. Neurosci. Abstr. 21:869.
Pellerin, L., and Magistretti, P.J. (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. U.S.A. 91:10625–10629.
Plum, F., and Hindfelt, B. (1976). The neurological complications of liver disease. In (P.J. Vinken and G.W. Bruyn, eds.) Handbook of Clinical Neurology, Vol. 27, North-Holland Publ., Amsterdam, pp. 349–377.
Qureshi., I.A., and Rao, K.V. (1996). Decreased brain cytochrome C oxidase activity in congenitally hyperammonemic spf mice: Effects of acetyl-L-carnitine. In (R.L. Mardini, ed.) Advances in Hepatic Encephalopathy and Metabolism in Liver disease, Ipswich Book Company, UK, pp. 385–393.
Qureshi, K., Rao, K.V., and Qureshi, I.A. (1998). Differential inhibition by hyperammonemia of the electron transport chain enzymes in synaptosomes and nonsynaptic mitochondria in ornithine transcarbamylase deficient spf-mice: Restoration by acetyl-L-carnitine. Neurochem. Res. 23:855–861.
Raabe, W.A. (1987). Synaptic transmission in ammonia intoxication. Neurochem. Pathol. 6:145–166.
Raabe, W.A. (1989). Neuropathology of ammonia intoxication. In (R.F. Butterworth and G. Pomier Layrargues, eds.) Hepatic Encephalopathy: Pathophysiology and Treatment, Humana Press, Clifton, NJ, pp. 49–77.
Rao, V.L., Audet, R., and Butterworth, R.F. (1995). Increased neuronal nitric oxide synthase activities and [3H]-arginine uptake in brain following porta-caval anastomosis. J. Neurochem. 65:677–681.
Rao, V.L., Audet, R., and Butterworth, R.F. (1997). Porta-caval shunting and hyperammonemia increases [3H]-arginine uptake but not L-[3H]-nitroarginine uptake into rat brain synaptosomes. J. Neurochem. 68:337–343.
Rao, K.V., and Qureshi, I.A. (1997). Progressive decrease of cerebral cytochrome C oxidase activity in sparse-fur mice: Role of acetyl-L-carnitine in restoring ammonia-induced cerebral energy depletion. Neurosci. Lett. 224:83–86.
Ratnakumari, L., Audet, R., Qureshi, I.A., and Butterworth, R.F. (1995b). NaC-KC-ATPase activities are increased in both congenital and acquired hyperammonemic syndromes. Neurosci. Lett. 197:89–92.
Ratnakumari, L., and Murthy, Ch.R.K. (1992a). In vitro and in vivo effects of ammonia on glucose metabolism in the astrocytes of rat cerebral cortex. Neurosci. Lett. 148:85–88.
Ratnakumari, L., and Murthy, Ch.R.K. (1992b). Response of rat cerebral glycolytic enzymes to hyperammonemic states. Neurosci. Lett. 161:37–40.
Ratnakumari, L., and Murthy, Ch.R.K. (1989). Activities of pyruvate dehydrogenase, enzymes of citric acid cycle, and aminotransferases in the subcellular fractions of cerebral cortex in normal and hyperammonemic rats. Neurochem. Res. 14:221–223.
Ratnakumari, L., and Murthy, Ch.R.K. (1993). Response of rat cerebral glycolytic enzymes to hyperammonemia. Neurosci. Lett. 161:37–40.
Ratnakumari, L., Qureshi, I.A., and Butterworth, R.F. (1992). Effect of congenital hyperammonemia on cerebral and hepatic levels of intermediates of energy metabolism in spf mice. Biochem. Biophys. Res. Commun. 184:746–751.
Ratnakumari, L., Qureshi, I.A., and Butterworth, R.F. (1993). Effect of sodium benzoate on the cerebral and hepatic energy metabolites in spf mice with congenital hyperammonemia. Biochem. Pharmacol. 45:137–146.
Ratnakumari, L., Qureshi, I.A., and Butterworth, R.F. (1994). Regional amino acid neurotransmitter changes in brains of spf/Y mice with congenital ornithine transcarbamylase deficiency. Metab. Brain Dis. 9:43–51.
Ratnakumari, L., Qureshi, I.A., Maysinger, D., and Butterworth, R.F. (1995a). Developmental deficiency of the cholinergic system in congenitally hyperammonemic spf mice: Effect of acetyl-L-carnitine. J. Pharmacol. Exp. Ther. 274:437–443.
Ratnakumari, L., Subbalakshmi, G.Y.C.V., and Murthy, Ch.R.K. (1986). Acute effects of ammonia on the enzymes of citric acid cycle in rat brain. Neurochem. Intl. 8:115–120.
Record, C.O. (1991). Neurochemistry of hepatic encephalopathy. Gut 32:1261–1263.
Schousboe, A., Westergaard, N., Waagepetersen, H.S., Larsson, O.M., Bakken, I.J., and Sonnewald, U. (1997). Trafficking between glia and neurons of TCA cycle intermediates and related metabolites. Glia 21:99–105.
Stewart, V.C., Sharpe, M.A., Clark, J.B., and Heales, S.J.R. (2000). Astrocyte-derived nitric oxide causes both reversible and irreversible damage to the neuronal mitochondrial respiratory chain. J. Neurochem. 75:694–700.
Walshe, J.M., Decarli, L., and Davidson, C.S. (1958). Some factors influencing cerebral oxidation in relation to hepatic coma. Clin. Sci. Lond. 17:11–25, 27-36.
Waniewski, R.A. (1992). Physiological levels of ammonia regulate glutamine synthesis from extracellular glutamate in astrocyte cultures. J. Neurochem. 58:167–174.
Watanabe, H., and Passonneau, J.M. (1973). Factors affecting the turnover of cerebral glycogen and limit dextrin in vivo. J. Neurochem. 20:1543–1554.
Wendon, J.A., Harrison, P.M., Keays, R., and Williams, R. (1994). Cerebral blood flowand metabolism in fulminant hepatic failure. Hepatology 19, 1407–1413.
Worcel, A., and Erecinska, M. (1962). Mechanism of inhibitory action of ammonia on the respiration of rat-liver mitochondria. Biochem. Beefiest. Acta 65:27–33.
Yu, A.C.H., Schousboe, A., and Hertz, L. (1982). Metabolic fate of 14C-labelled glutamate in astrocytes in primary cultures. J. Neurochem. 39:954–960.
Zamora, A.J., Cavanaugh, J.B., and Kyu, M.H. (1973). Ultrastructural responses of the astrocytes to porta-caval anastomosis in the rat. J. Neurol. Sci. 18:25–45.
Zoratti, M., and Szabo, I. (1995). The mitochondrial permeability transition. Biochim. Biophys. Acta 1241:139–176.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rao, K.R., Norenberg, M. Cerebral Energy Metabolism in Hepatic Encephalopathy and Hyperammonemia. Metab Brain Dis 16, 67–78 (2001). https://doi.org/10.1023/A:1011666612822
Issue Date:
DOI: https://doi.org/10.1023/A:1011666612822