Abstract
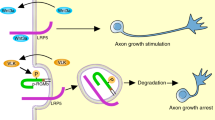
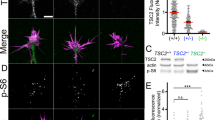
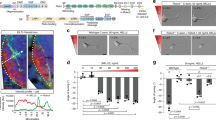
Tuberous sclerosis complex is a disease caused by mutations in the TSC1 or TSC2 genes, which encode a protein complex that inhibits mTOR kinase signaling by inactivating the Rheb GTPase. Activation of mTOR promotes the formation of benign tumors in various organs and the mechanisms underlying the neurological symptoms of the disease remain largely unknown. We found that Tsc2 haploinsufficiency in mice caused aberrant retinogeniculate projections that suggest defects in EphA receptor–dependent axon guidance. We also found that EphA receptor activation by ephrin-A ligands in neurons led to inhibition of extracellular signal–regulated kinase 1/2 (ERK1/2) activity and decreased inhibition of Tsc2 by ERK1/2. Thus, ephrin stimulation inactivates the mTOR pathway by enhancing Tsc2 activity. Furthermore, Tsc2 deficiency and hyperactive Rheb constitutively activated mTOR and inhibited ephrin-induced growth cone collapse. Our results indicate that TSC2-Rheb-mTOR signaling cooperates with the ephrin-Eph receptor system to control axon guidance in the visual system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Crino, P.B., Nathanson, K.L. & Henske, E.P. The tuberous sclerosis complex. N. Engl. J. Med. 355, 1345–1356 (2006).
Wong, M. Mechanisms of epileptogenesis in tuberous sclerosis complex and related malformations of cortical development with abnormal glioneuronal proliferation. Epilepsia 49, 8–21 (2008).
Manning, B.D., Tee, A.R., Logsdon, M.N., Blenis, J. & Cantley, L.C. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell 10, 151–162 (2002).
Inoki, K., Li, Y., Zhu, T., Wu, J. & Guan, K.L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signaling. Nat. Cell Biol. 4, 648–657 (2002).
Flanagan, J.G. Neural map specification by gradients. Curr. Opin. Neurobiol. 16, 59–66 (2006).
Feldheim, D.A. et al. Topographic guidance labels in a sensory projection to the forebrain. Neuron 21, 1303–1313 (1998).
Pfeiffenberger, C. et al. Ephrin-As and neural activity are required for eye-specific patterning during retinogeniculate mapping. Nat. Neurosci. 8, 1022–1027 (2005).
Pfeiffenberger, C., Yamada, J. & Feldheim, D.A. Ephrin-As and patterned retinal activity act together in the development of topographic maps in the primary visual system. J. Neurosci. 26, 12873–12884 (2006).
Torborg, C.L. & Feller, M.B. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog. Neurobiol. 76, 213–235 (2005).
Pasquale, E.B. Eph-ephrin bidirectional signaling in physiology and disease. Cell 133, 38–52 (2008).
Lin, A.C. & Holt, C.E. Function and regulation of local axonal translation. Curr. Opin. Neurobiol. 18, 60–68 (2008).
Brittis, P.A., Lu, Q. & Flanagan, J.G. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell 110, 223–235 (2002).
Leung, K.M. et al. Asymmetrical beta-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat. Neurosci. 9, 1247–1256 (2006).
Wu, K.Y. et al. Local translation of RhoA regulates growth cone collapse. Nature 436, 1020–1024 (2005).
Yao, J., Sasaki, Y., Wen, Z., Bassell, G.J. & Zheng, J.Q. An essential role for beta-actin mRNA localization and translation in Ca2+-dependent growth cone guidance. Nat. Neurosci. 9, 1265–1273 (2006).
Campbell, D.S. & Holt, C.E. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron 32, 1013–1026 (2001).
Choi, Y.J. et al. Tuberous sclerosis complex proteins control axon formation. Genes Dev. 22, 2485–2495 (2008).
Onda, H., Lueck, A., Marks, P.W., Warren, H.B. & Kwiatkowski, D.J. Tsc2+/− mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J. Clin. Invest. 104, 687–695 (1999).
Torborg, C.L. & Feller, M.B. Unbiased analysis of bulk axonal segregation patterns. J. Neurosci. Methods 135, 17–26 (2004).
Stevens, B. et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178 (2007).
Ellsworth, C.A., Lyckman, A.W., Feldheim, D.A., Flanagan, J.G. & Sur, M. Ephrin-A2 and -A5 influence patterning of normal and novel retinal projections to the thalamus: conserved mapping mechanisms in visual and auditory thalamic targets. J. Comp. Neurol. 488, 140–151 (2005).
Huberman, A.D., Murray, K.D., Warland, D.K., Feldheim, D.A. & Chapman, B. Ephrin-As mediate targeting of eye-specific projections to the lateral geniculate nucleus. Nat. Neurosci. 8, 1013–1021 (2005).
Sahin, M. et al. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron 46, 191–204 (2005).
Di Nardo, A. et al. Tuberous sclerosis complex activity is required to control neuronal stress responses in an mTOR-dependent manner. J. Neurosci. 29, 5926–5937 (2009).
Miao, H. et al. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat. Cell Biol. 3, 527–530 (2001).
Willis, D.E. et al. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J. Cell Biol. 178, 965–980 (2007).
Aakalu, G., Smith, W.B., Nguyen, N., Jiang, C. & Schuman, E.M. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron 30, 489–502 (2001).
Yudin, D. et al. Localized regulation of axonal RanGTPase controls retrograde injury signaling in peripheral nerve. Neuron 59, 241–252 (2008).
Roche, F.K., Marsick, B.M. & Letourneau, P.C. Protein synthesis in distal axons is not required for growth cone responses to guidance cues. J. Neurosci. 29, 638–652 (2009).
Ma, L., Chen, Z., Erdjument-Bromage, H., Tempst, P. & Pandolfi, P.P. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121, 179–193 (2005).
Torii, M., Hashimoto-Torii, K., Levitt, P. & Rakic, P. Integration of neuronal clones in the radial cortical columns by EphA and ephrin-A signaling. Nature 461, 524–528 (2009).
Filosa, A. et al. Neuron-glia communication via EphA4/ephrin-A3 modulates LTP through glial glutamate transport. Nat. Neurosci. 12, 1285–1292 (2009).
Ehninger, D. et al. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat. Med. 14, 843–848 (2008).
Benson, M.D. et al. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc. Natl. Acad. Sci. USA 102, 10694–10699 (2005).
Park, K.K. et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322, 963–966 (2008).
Yang, Q., Inoki, K., Kim, E. & Guan, K.L. TSC1/TSC2 and Rheb have different effects on TORC1 and TORC2 activity. Proc. Natl. Acad. Sci. USA 103, 6811–6816 (2006).
Piper, M. et al. Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron 49, 215–228 (2006).
Merianda, T.T. et al. A functional equivalent of endoplasmic reticulum and Golgi in axons for secretion of locally synthesized proteins. Mol. Cell. Neurosci. 40, 128–142 (2009).
Bolton, P.F., Park, R.J., Higgins, J.N., Griffiths, P.D. & Pickles, A. Neuro-epileptic determinants of autism spectrum disorders in tuberous sclerosis complex. Brain 125, 1247–1255 (2002).
Weber, A.M., Egelhoff, J.C., McKellop, J.M. & Franz, D.N. Autism and the cerebellum: evidence from tuberous sclerosis. J. Autism Dev. Disord. 30, 511–517 (2000).
Wong, V. & Khong, P.L. Tuberous sclerosis complex: correlation of magnetic resonance imaging (MRI) findings with comorbidities. J. Child Neurol. 21, 99–105 (2006).
Volpe, J.J. Neuronal proliferation, migration, organization and myelination. in Neurology of the Newborn (ed. Volpe, J.J.) 45–99 (W.B. Saunders, Philadelphia, 2001).
Sutula, T., Cascino, G., Cavazos, J., Parada, I. & Ramirez, L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann. Neurol. 26, 321–330 (1989).
Courchesne, E. et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 57, 245–254 (2001).
Barnea-Goraly, N. et al. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol. Psychiatry 55, 323–326 (2004).
Henske, E.P. et al. Allelic loss is frequent in tuberous sclerosis kidney lesions but rare in brain lesions. Am. J. Hum. Genet. 59, 400–406 (1996).
Tavazoie, S.F., Alvarez, V.A., Ridenour, D.A., Kwiatkowski, D.J. & Sabatini, B.L. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat. Neurosci. 8, 1727–1734 (2005).
Goorden, S.M., van Woerden, G.M., van der Weerd, L., Cheadle, J.P. & Elgersma, Y. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Ann. Neurol. 62, 648–655 (2007).
Shamah, S.M. et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell 105, 233–244 (2001).
Hernandez, O., Way, S., McKenna, J. III & Gambello, M.J. Generation of a conditional disruption of the Tsc2 gene. Genesis 45, 101–106 (2007).
Acknowledgements
We thank B. Stevens and D. Shafer for assistance with multi-threshold LGN analysis, J. Twiss and T. Merianda for assistance with fluorescence recovery after photobleaching and M. Bear, E. Osterweil and D. Krueger for assistance with metabolic labeling experiments. The Tsc2loxP/loxP mice were a gift from M. Gambello, and the Tsc1loxP/loxP mice were gift of D. Kwiatkowski. We are also grateful to B. Stevens, C. Chen, Z. He and members of the Sahin laboratory for critical reading of the manuscript and to L. Mariani, A. Sadowski and S. Goldman for technical assistance. This work was supported in part by grants from the US National Institutes of Health (NS58956 to M.S. and HD025938 to E.B.P.), the John Merck Scholars Fund, Tuberous Sclerosis Alliance, the Manton Foundation and Children's Hospital Boston Translational Research Program to M.S. and the Children's Hospital Boston Mental Retardation and Developmental Disabilities Research Center (P01 HD18655). D.N. is supported by a Mentor Based Postdoctoral Fellowship from Autism Speaks. A.D.N. is supported by a grant from the Hearst Foundation. H.B. is supported by an Howard Hughes Medical Institute Research Training Fellowship for Medical Students.
Author information
Authors and Affiliations
Contributions
D.N. performed most of the experiments. A.D.N. carried out the preliminary biochemistry experiments on the relationship between Eph and Tsc pathways. D.N., I.K., T.H. and M.S. conducted and analyzed the retinal projection experiments. J.M.H. and H.B. performed the quantification on the growth cone collapse assays and immunocytochemical staining. S.D. provided guidance with the initial experiments on the Tsc2+/− mice. P.P.P. provided reagents and troubleshooting for experiments on Tsc2 Ser664 phosphorylation. S.C. and E.B.P. contributed unpublished preliminary data on the Eph-Tsc interaction. M.S. supervised the project. D.N., E.B.P. and M.S. wrote and edited the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14 (PDF 7835 kb)
Rights and permissions
About this article
Cite this article
Nie, D., Di Nardo, A., Han, J. et al. Tsc2-Rheb signaling regulates EphA-mediated axon guidance. Nat Neurosci 13, 163–172 (2010). https://doi.org/10.1038/nn.2477
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2477
This article is cited by
-
The role of TSC1 and TSC2 proteins in neuronal axons
Molecular Psychiatry (2024)
-
RHOA signaling defects result in impaired axon guidance in iPSC-derived neurons from patients with tuberous sclerosis complex
Nature Communications (2021)
-
Autophagy and apoptosis cascade: which is more prominent in neuronal death?
Cellular and Molecular Life Sciences (2021)
-
Revisiting Brain Tuberous Sclerosis Complex in Rat and Human: Shared Molecular and Cellular Pathology Leads to Distinct Neurophysiological and Behavioral Phenotypes
Neurotherapeutics (2021)
-
Targeted therapy for mTORC1-driven tumours through HDAC inhibition by exploiting innate vulnerability of mTORC1 hyper-activation
British Journal of Cancer (2020)