Abstract
Neonates who present in high output heart failure secondary to vein of Galen aneurysmal malformation can be difficult to manage medically due to the complex physiology that results from the large shunt through the malformation. Though the cardiac function is often normal, right ventricular dilation, severe pulmonary hypertension, and systemic steal can result in inadequate organ perfusion and shock. This report recommends medical management for stabilization of neonates prior to definitive management with endovascular embolization.
Impact
-
Vein of Galen aneurysmal malformation (VGAM) is a rare intracranial arteriovenous malformation, which can present in the neonatal period with high output heart failure.
-
Heart failure secondary to VGAM is often difficult to manage and is associated with high mortality and morbidity. Despite optimal medical management, many patients require urgent endovascular embolization for stabilization of their heart failure.
-
This report offers discrete recommendations that can be used by clinicians as guidelines for the medical management of heart failure in newborns with VGAM.
Similar content being viewed by others
Introduction
Vein of Galen aneurysmal malformation (VGAM), a rare congenital intracranial arteriovenous (AV) malformation (AVM) of the cerebral vasculature, represents about 30% of all prenatally diagnosed intracranial AV anomalies and has an incidence of 1/25,000 births. There are two major anatomic subtypes, the choroidal and the mural types, which embryologically result from abnormal connections between the primitive choroidal vessels and the prosencephalic vein of Markowski, occurring between the 6th and 11th weeks of gestation.1
The exact pathogenesis remains unclear. Mechanical reasons, such as thrombosis, have been speculated to precede the development of the AVM.1,2 Recently, genetic factors have been shown to be present in up to 30% of these patients.3,4
Neonates with VGAM have a spectrum of signs related to high output heart failure (HOHF). On one end of the spectrum, neonates can have minimal signs that can be medically managed until 5–6 months of age when endovascular embolization can be performed. On the other end of the spectrum, neonates can present in cardiogenic shock that is difficult to manage medically and requires urgent endovascular intervention unless they are deemed too high risk for intervention. Fetuses with large VGAM often do not develop signs of HF in utero because the low resistance of the AVM is balanced by the low vascular resistance in the placenta.5,6,7
The goal of this report is to recommend guidelines for the medical management of infants with VGAM based on our review of the literature and the experience of our institutions. Emphasis is given to the severe forms of VGAM that present with early HOHF and need endovascular treatment urgently. It is important to note that, due to the rarity of this condition and the lack of randomized studies on the optimum pharmacologic treatments, there is variability in the management of these patients.
Methods
These guidelines represent a combination of review of the literature and the experience of our institutions (Bicêtre Medical Centre, a high volume referral center in France, and the University of Texas Southwestern Medical Center/Children’s Health, a Level IV program in the USA). For the literature search, we attempted a comprehensive search pertaining to VGAM. We searched Pubmed and OVID Medline with the following terms: “vein of Galen malformation,” “intracranial arteriovenous malformation,” “cerebral arteriovenous malformation,” “heart failure,” “cardiac output, high.” Due to the limited number of research articles addressing the management of neonates with vein of Galen malformation, many of the recommendations are extrapolated from other disease process and are noted throughout these guidelines.
Defining and understanding the physiology of VGAM
Of the neonates who develop HOHF from VGAM, most will present during the first week of life.8 The pathophysiology of the HF is secondary to the increased blood flow through the low resistance vessels of the AVM. This results in increased return via the superior vena cava (SVC) and a large volume load to the right ventricle (RV). SVC flows up to 10-fold greater than normal have been reported.9 These neonates often have elevated pulmonary artery (PA) pressure which is a direct result of the increased pulmonary blood flow (PBF), resulting in an increased afterload to the RV. There is some evidence that vascular remodeling occurs in utero that contributes to the degree of pulmonary hypertension (PH) in these patients.10,11 The most severe of these patients will have suprasystemic PA pressure.
The combined increased volume load and increased afterload to the RV results in a distended and non-compliant RV with shift of the interventricular septum to the left. The left ventricle (LV) often has normal or hyperdynamic function; however, the cardiac output (CO) is unable to meet the metabolic demand of the systemic organs resulting in lactic acidosis. In addition, the low resistance AVM results in “steal” from the lower body and decreased perfusion to the vital organs. Almost 30% of infants demonstrate evidence of myocardial ischemia from decreased coronary perfusion.12 Total systemic vascular resistance (SVR) is expected to be low secondary to the low resistance from the VGAM; however, extracranial SVR is elevated due to compensatory changes from decreased perfusion to the body (physiology summarized in Fig. 1).
a Physiology and blood flow in a normal heart with a closed ductus arteriosus. b Physiology and blood flow in a neonate with a vein of Galen aneurysmal malformation (VGAM) with an open ductus arteriosus and in high output heart failure.  arterial blood flow,
arterial blood flow,  venous blood flow,
venous blood flow,  VGAM shunt. DA ductus arteriosus, LA left atrium, LPA left pulmonary artery, LV left ventricle, IVC inferior vena cava, IVS interventricular septum, RA right atrium, RPA right pulmonary artery, RV right ventricle, SVC superior vena cava.
VGAM shunt. DA ductus arteriosus, LA left atrium, LPA left pulmonary artery, LV left ventricle, IVC inferior vena cava, IVS interventricular septum, RA right atrium, RPA right pulmonary artery, RV right ventricle, SVC superior vena cava.
There is a high morbidity and mortality related to the development of HF in the neonatal period, with early mortality estimated at 20–50%.12 Suprasystemic PA pressure and severe congestive HF requiring mechanical ventilation have both been associated with poor outcomes.8,13 Infants with VGAM can have associated congenital heart defects, including sinus venosus atrial septal defects (ASD), partial anomalous pulmonary venous return (PAPVR), ventricular septal defects (VSD) and coarctation of the aorta, which further complicate management and ouctomes.9,12,14
Antenatal factors that can predict aggressiveness of postnatal heart failure
Although the diagnosis of VGAM can be made prenatally, it can be difficult to predict which infants will have the most significant disease postnatally. The choroidal subtype (consisting of multiple, bilateral AV connections penetrating the prosencephalic varix) is associated with severe neonatal disease more frequently than the mural subtype (consisting of a single AV fistula).15 These findings are also influenced by the exact anatomy of each type and cannot be completely predicted by in utero studies.16 Encephalomalacia, if seen in utero, is universally associated with severe early HF.
By antenatal magnetic resonance imaging (MRI), VGAM varix volume of >20,000 mm3,17 larger measurements of the narrowest portion of the straight and falcine sinus18 and middle cerebral artery pseudofeeders19 have been identified as risk factors for the occurrence of encephalomalacia and poor outcomes. However, routine ability of neuroradiologists for standardized assessment of these lesions has been questioned.18
Small studies have identified findings on fetal echocardiogram that may be associated with neonatal mortality, including greater than mild tricuspid regurgitation, greater than mild RV dysfunction, and higher calculated cardiac index.17,20
Due to lack of standardization, we caution the use of antenatal findings as predictors for aggressiveness of clinical course.
Hemodynamic factors and conditions that could modify timing of presentation
Infants with VGAM can be relatively stable at birth and present after the first 24 h of life, with the median time of presentation being 3 days of life.8 The variability of the deterioration in these patients is difficult to predict.
Immediately after birth, the cardiovascular system adapts to (1) decreasing pulmonary vascular resistance (PVR), (2) the closing of the ductus arteriosus (DA) and (3) increasing SVR. All of these physiologic phenomena could contribute to a degree to deterioration in a patient with VGAM with some significant differences.
Normally, the PVR and PA pressures decrease over time as the lungs adapt to breathing physiology and increasing oxygen tension. This transition has not been adequately studied in patients with VGAM; however, it is possible that the vasodilation of the pulmonary vasculature, resulting in a rapid and large volume of PBF, is exacerbated by the large left-to-right shunt from the VGAM. This increased PBF may contribute to further rapid decrease in inherent PVR. In different disease processes, increased PBF and shear stress are associated with lung vascular nitric oxide (NO) and prostacyclin production resulting in vasodilation and lower PVR.21,22,23,24 Consequently, neonates with VGAM may recruit their pulmonary capillary bed quickly after birth and have low inherent PVR. The PBF is obligatory due to the large left-to-right shunt at the level of the AVM which results in elevated PA pressure from high PBF despite a low PVR (Ohm’s law: pressure = flow × resistance). The timing of the rise in PA pressures may contribute to the variability of the clinical presentation in neonates with VGAM.
The constricting DA affects RV afterload and likely also contributes to the timing of presentation. In patients with severe PH, the patent DA allows right-to-left shunting prior to relaxation of the pulmonary vascular bed which can help maintain CO and provide a “pop-off” for the pressure-loaded RV.
SVR increases in the immediate postnatal period secondary to birth-related endogenous catecholamine release. This rapid change in SVR may affect the shunting through the AVM contributing to the development of HOHF after birth. It is theorized that, in utero, the low resistance AVM is balanced by the low resistance placenta. Once the low resistance placenta is removed and the SVR rises through normal transition, there is likely a change in the blood flow dynamics in neonates with VGAM.
SVC flow has been calculated to be 40–120 mL/kg/minute immediately after birth in normal newborns, which then increases over the next 24–48 h to 250 ml/kg/min.9,25,26 This rate of expected SVC blood flow increase after birth has not been described in newborns with VGAM, but could contribute to the pathophysiology of the disease and the associated clinical deterioration. One case series presented echocardiogram data describing SVC flows up to 800 ml/kg/min (7-8 times normal) in neonates with VGAM who presented early with HF.9
Associated congenital heart defects may also affect hemodynamics and timing of presentation. One patient in a case series with a large sinus venosus ASD presented at 3 months of life with no significant acidosis and balanced, but increased output from the RV and LV.9 The role of the large unrestricted ASD was not discussed by the authors as a cause for the relative stability of this patient for the first 3 months of life but, in theory, could have helped equilibrate the increased flow at the atrial level and balance the effect of the VGAM. In neonates with VGAM, the RV end-diastolic pressure and right atrial pressure is high from the increased volume load, and hence a large ASD would allow right-to-left shunting and offloading volume from the RV.
Echocardiography parameters for the prediction of severity of disease
There is interest in echocardiography to predict which neonates will have severe HF at an early age. Several small single center studies have tried to identify specific parameters that can be used.
The ratio of antegrade-to-retrograde flow in the aortic arch based on velocity time integrals can potentially describe the degree of “steal” by the AVM. One case series suggested a ratio of antegrade-to-retrograde flow <1.5 in the first postnatal echocardiogram may be predictive of mortality.27 In another case series, a post-intervention diastolic flow velocity of >20% of the systolic velocity is not well tolerated and suggests need for additional neurointervention.28 Additionally, another case series described retrograde flow in the descending aorta extending into systole being associated with an adverse outcome.29 A relatively large cohort of patients also demonstrated mortality association with retrograde flow in the descending aorta of >0.3 m/s.12
SVC flow has also been identified as a potential marker for severity of disease as it reflects the volume of the venous return from the AVM. One case series suggested that SVC flow >400 ml/kg is associated with an adverse outcome despite early embolization.29 SVC inflow to LV outflow ratio has also been described as a predictor of poor outcome.12
Signs of suprasystemic PA pressure at presentation are associated with poor prognosis.10 Suprasystemic PA pressure should not be considered a contraindication to intervention, but may signify the need for early intervention.30 Ventricular dysfunction should also be considered a sign of poor prognosis.
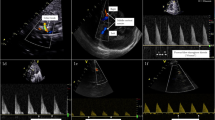
Echocardiography findings in patients with HOHF from VGAM are compared to normal patients in Fig. 2.
a 2D images in the apical four chamber, parasternal short axis, and subcostal sagittal views are included side by side in a comparative way (normal left, abnormal right). b Doppler images in the parasternal long axis are included side by side (normal left, abnormal right). VTI velocity–time integral.
Rationale for the management of VGAM
Role of vasoactive support
Currently, there are no randomized studies for use of inotropic support with this lesion. Thus, the data rely on retrospective cohorts, case series and case reports. Even in centers that have reported outcomes, the choice of vasoactive medications is not standardized and in some cases appear to have changed over time. This has made evaluating the available data difficult as there is often contradictory information. It is also worth noting, that it can be difficult to extrapolate published data on inotropes in adults and in children with other types of shock, as the pathophysiology differs in neonates with VGAM. As a result, choosing a vasoactive medication is often based on known physiology of the lesion, the expected pharmacology of the drug and the combined evaluation of the available literature. In Table 1, we summarize the known effects of each vasoactive medications reported to date. It is expected that small changes in dosing or combinations of these vasoactive medications could result in profound changes in SVR/PVR in patients with VGAM.
Dopamine
Historically, dopamine, an endogenous catecholamine and precursor of norepinephrine, has been used for inotropic support of patients with VGAM. It exerts its cardiovascular actions through the dose-dependent stimulation of dopaminergic, α- and β-adrenergic receptors with significant renal and endocrine effects.31 The cardiovascular effect of dopamine is mediated at least in part by the stimulation of Dopamine 2 receptors in the sympathetic neurons of the myocardium to release endogenous NE, which in turn results in the activation of adrenergic receptors (the “indirect” effect).31,32,33 In newborns, these stores are immature, poorly developed and rapidly depleted after use,34 resulting in resistance towards dopamine.35,36,37
In neonates, dopamine has a multi-phasic vasoactive effect.38 Although there is a possible overlap for doses of 4–5 and 8–10 mcg/kg/minute between α and β effects, low doses of dopamine should be considered to further increase SVR, a relatively paradoxical effect that might not necessarily be warranted in VGAM patients. Hence, it is recommended to use low to intermediate doses (5–10 mcg/kg/min) targeting β-adrenergic effects. This would assure increase in cardiac contractility, without significant increases in SVR. If a significant chronotropic effect occurs, then down titration of dopamine would be recommended. Increasing doses of dopamine result in increases on both PVR and SVR. At low doses, SVR is affected more than PVR, while at high doses, PVR is affected more than SVR.39,40,41
Epinephrine
The effects of epinephrine, an endogenous catecholamine, depend on the dose administered. At low doses (0.02–0.05 mcg/kg/min), epinephrine has effects that are similar to those of dopamine at similar low and moderate doses. Beta-agonist effects predominate with enhanced myocardial contractility, some peripheral vasodilation, while PVR possibly decreases. High doses (>0.1 mcg/kg/min) have predominately α-adrenergic effects which cause increased peripheral vasoconstriction, resulting in increased afterload which could lead to increased cardiac work and oxygen consumption (VO2).42 At these dose ranges, cardiovascular side effects can be observed and include tachycardia, systemic hypertension as well as lactic acidosis due to potent cellular metabolic effects from activation of glycogenolysis, a β2-adrenergic action. Doses above 0.5 mcg/kg/min should be avoided due to the inadverdent increase in afterload and decrease in CO.
Norepinephrine
Norepinephrine, a potent α1 agonist with some β1 and minimal β2 activity, stimulates elevation of SVR, minimal chronotropic and some inotropic effects. As explained earlier, norepinephrine is the active end mediator of dopamine, with no effects on dopaminergic receptors. The usual doses in newborns range between 0.01-0.05 mcg/kg/min and the net effect results in elevation of blood pressure with no significant tachycardia. Norepinephrine can improve coronary blood flow by raising diastolic blood pressure (BP) and through direct coronary dilation.43,44 The most concerning side effect is compromised organ blood flow; BP may increase without improved perfusion.44
Dobutamine
Dobutamine, a relatively cardioselective synthetic catecholamine, has two enantiomeric forms with a net effect of primarily β1 agonism with minimal β2 and α effects. It exerts its effects via direct stimulation of myocardial adrenergic receptors, and does not rely on myocardial norepinephrine stores for action. Its effect in improving CO and SVC flows is evident within 30 min from initiation of moderate doses, even in preterm newborns.45 Dobutamine is also associated with a variable decrease in SVR via action on peripheral β2 receptors, although this effect most likely occurs at higher doses.46,47 In adults, these effects result in improved coronary blood flow and myocardial function.48 Major side effect concerns are tachycardia and arrhythmias. At low doses, there is inotropy with minimal chronotropy, but at higher doses, there is more heart rate effect. However, even at low doses without significant heart rate effect, there is a significant increase in myocardial VO2. In recent literature, dobutamine has been used as monotherapy for inotropic support in VGAM, and is considered the drug of choice by some centers.7,30 On the other hand, the increased myocardial VO2 and tachycardia could result in clinical deterioration by decreasing coronary diastolic filling time and myocardial ischemia.
Milrinone
Milrinone, a type 3 phosphodiesterase (PDE) inhibitor, improves inotropy and lusitropy by preventing intracellular cAMP degradation. Milrinone also inhibits cAMP degradation in the peripheral endothelium which results in peripheral vasodilatation of the pulmonary and systemic vasculature. PDE3 inhibitors increase cardiac muscle contractility and CO without increasing myocardial oxygen demand. Data from immature animals, though, show that PDE3 inhibitors might not be effective immediately after birth49,50,51 which is secondary to a transient maturational deficit in the sarcoplasmic reticulum PDE3.52 PDE3 is upregulated after birth, with an associated increase in the efficacy of its inhibitors as early as 12 h of life.53 Milrinone improves CO and oxygen delivery in infants after congenital heart surgery.54 In adults with congestive HF, milrinone can decrease myocardial VO2 by decreasing coronary vascular resistance and myocardial oxygen extraction.55
Milrinone, in theory, would represent an ideal medication for neonates with VGAM as it improves systolic and diastolic function, produces systemic and pulmonary vasodilation and matches coronary arterial supply and demand. For this reason, it has been used either as monotherapy or as adjunctive medication in neonates with VGAM with mixed results.5,9,12,30 There have been reports that demonstrated improved systemic arterial pressure and systemic perfusion with the addition of milrinone.5,30
The most concerning side effect is hypotension due to its vasodilatory properties. If milrinone is used for patients with VGAM, starting at low dose (0.25 mcg/kg/min) without a loading dose would be recommended to avoid increased risk of hypotension and accumulation in the setting of possible decreased clearance.56,57 In hypotensive newborns with VGAM, stabilization of BP with another vasoactive prior to use of milrinone is recommended.
Levosimendan
Levosimendan, a calcium sensitizer, provides inotropic support by binding to Troponin C and increasing sensitivity to calcium, thereby improving myocardial contractility. It also causes vasodilation by opening ATP-dependent potassium channels. The primary benefit to this class of medication is that it results in improved cardiac function without increasing intracellular calcium or myocardial VO2.43 Levosimendan is not currently available in the United States, but has been used successfully in many other countries in different patient populations. Additionally, it has been reported in neonates with VGAM with some success, though the data remain limited.30
Vasopressin
Vasopressin has a well described peripheral vasoconstrictive effect (via V1 receptors), osmoregulation (via V2 receptors in the collecting tubules) and corticotropin secretion (via V3 receptors in the CNS).58,59 Limited neonatal and pediatric studies suggest dose ranges of 0.17–8 milliunits/kg/min (0.01–0.48 units/kg/h). Vasopressin also affects various components of the prostaglandin and NO pathway which could explain some of its peripheral vasodilatory effects in the kidney, lung, coronaries and brain.60,61 Patients with catecholamine-resistant shock respond to vasopressin,62 but with no significant reduction in mortality.63 One specific finding in the meta-analysis that may be beneficial for neonates with VGAM is a significant reduction in heart rate after the addition of vasopressin. In our experience (unpublished data), vasopressin was used in a rapidly deteriorating patient with VGAM with relative stabilization of the BP, but with no improvement in the lactic acidemia or overall clinical status. Terlipressin, a vasopressin analog, has been used in three patients with VGAM. Two survived with no evidence of stroke in MRI.30
Due to lack of evidence and potential side effects, we do not recommend routine use of vasopressin as a first-line agent in patients with VGAM.
Summary of catecholamines
Dopamine is the most commonly used vasoactive medication in the neonatal intensive care unit (60–70%) and the use of dobutamine has declined.64 At low doses, when compared to dobutamine, dopamine induces less chronotropic effect.47 Higher doses in both drugs have similar chronotropic effects. A direct comparison of dopamine with epinephrine (both at low/moderate doses) in neonates showed that there were no significant differences in basic hemodynamics, while HR increased more in patients treated with epinephrine.65 Comparison of catecholamines in adult patients demonstrated higher mortality and incidence of arrhythmia with dopamine, and no difference in mortality between norepinephrine and epinephrine.66
Experience from the Bicêtre Centre has shifted the paradigm in the use of inotropic support. In 2002, use of dobutamine in addition to dopamine was reported with relatively unfavorable outcomes in several patients.10 In a subsequent study, norepinephrine was used as a primary inotrope in order to restore coronary blood flow with the addition of milrinone in some cases for RV support. While there was no report of outcomes based on use of inotropes, only five of 77 newborns died of uncontrollable HF with an overall mortality of 33%. Indeed, 41% of the survivors had a good global outcome.12
Catecholamines provide variable levels of inotropy, chronotropy and peripheral vasoconstriction. All catecholamine agents have the potential to induce tachycardia and increase myocardial VO2, negative effects that could outweigh any benefit to the failing RV from inotropic support. We recommend using low dose dopamine or epinephrine with close monitoring of HR or using NE which is less likely to result in tachycardia.
Summary of inodilators
Inodilators, agents that provide both inotropy and peripheral vasodilation, may have a role in the management of patients with VGAM. These include milrinone, dobutamine and levosimendan.
In pediatric patients with acute decompensated HF, the use of milrinone has increased while the use of dobutamine has decreased in US hospitals.67 Randomized trials have shown no difference in mortality between levosimendan and dobutamine in pediatric acute decompensated HF,68 or between milrinone and dobutamine in adult cardiogenic shock.69 A propensity matched study demonstrated a survival advantage with vasopressor plus inodilator compared to vasopressor alone in adult cardiogenic shock.70 While the pathophysiology of cardiogenic shock and HOHF from VGAM are different, there may be a role in utilizing a combined therapy strategy. In several case reports, catecholamines have been used in combination with an inodilator in neonates with VGAM. The addition of levosimendan in one case and milrinone in another case to dopamine, dobutamine and epinephrine was associated with improved hemodynamics.30 In another case series, addition of an arterial vasodilator to a ß-agonist resulted in improved systemic perfusion.5
Based on this information, it would be reasonable to use an inodilator as a single agent in patients with normal BP. In patients that are hypotensive, the combination of a low dose catecholamine with an inodilator may be beneficial.
Management of PH
As discussed above, the increased PA pressures in patients with VGAM is most likely secondary to increased flow through the PAs and not elevated PVR.9 Because of this, PH with VGAM can be resistant to treatment with oxygen and nitric oxide (iNO).
Since most studies report underfilled LV with reduced LV preload, use of pulmonary vasodilators in an attempt to further decrease PVR, could improve return to the LA and improve LV preload while simultaneously reducing RV afterload. In one report, iNO was able to decrease the PA pressure.71 Despite this, there are many studies which do not show a reduction in PA pressure or clinical improvement with the use of iNO.12 In addition, concerns for increasing PBF and additional pulmonary edema have been raised.
Maintaining a patent DA with PGE1 can help sustain CO via right-to-left shunting prior to the pulmonary vascular bed in the setting of PH, albeit at the expense of hypoxemia. The DA also reduces the afterload on the pressure loaded RV by providing a “pop-off”. There is cardiac catheterization data to suggest that blood from the LV preferentially is routed to the cerebral circulation, while blood from the RV via the DA preferentially goes to the descending aorta.6 Quantification of blood flow in neonates with VGAM by echocardiogram contradicts this with blood from the DA flowing retrograde to the cerebral circulation.9 Case series demonstrate mixed survival outcomes with the use of PGE1 in neonates with VGAM.7,12,72
Because of the lack of evidence to suggest benefit, we do not recommend using high FiO2 or iNO routinely in these infants. It is reasonable to use iNO as a trial in infants with VGAM and severe RV hypertension, but should be discontinued if no clinical benefit is observed. As noted above, using PGE1 to maintain a patent DA may be beneficial. We recommend initiating PGE1 shortly after birth and monitoring closely. If there are any signs of additional steal from left-to-right shunt, then PGE1 should be discontinued. We also recommend weaning off PGE1 infusions within 24–48 h after the embolization, to prevent additional steal.
Fluid and electrolyte management
Early use of diuretic therapy has been reported in the literature for the management of the HF in VGAM patients after birth. Loop of Henle diuretics, such as furosemide, are the most commonly reported. The goal of diuretic therapy is to change the intravascular volume load, improve the Frank-Starling relationship and the overall function of the overloaded RV. Fluid restriction may be beneficial to prevent further volume overload and may decrease the amount of diuretics needed.
Optimization of arterial pH and serum ionized calcium concentrations is important for the cardiovascular response to catecholamines. Metabolic acidosis (pH <7.25) compromises myocardial function in newborns,73 and maintenance of the arterial pH above this is recommended. Decreasing pH secondary to poor organ perfusion will possibly result in loss of efficacy of inotropic medications and further rapid deterioration. With increasing acidosis despite medical management, escalation of care and early embolization is recommended. A summary of our recommended guidelines for medical management can be found in (Table 2).
Bicêtre score
The acuity score developed at the Bicêtre Centre8,15,30 provides a comprehensive, easy to calculate point system of key elements that are associated with deterioration.74,75 The Bicêtre score is an important and validated score, but has some limitations (Table 3). We caution against its use as a sole predictive tool, but rather should be considered in the broader clinical picture for decision-making in patients with VGAM.
Defining futility in VGAM
About 30% of newborns are not candidates for early embolization for the following reasons: (1) severe brain injury on postnatal MRI, and (2) concerns for severe cardiogenic shock or multiple organ failure despite optimization of management.12
Conclusion
This report provides guidance for the medical management of patients with VGAM. The primary goal of management is to improve effective CO and thus the delivery of oxygen to the vital organs. Based on the pathophysiology of the HF, this can be accomplished through supporting the failing RV while not further worsening the systemic “steal.” As explained earlier, the diastolic flow reversal secondary to the “steal” from the AVM results in decreased perfusion of the extracranial organs which results in elevated SVR to maintain perfusion pressure of these organs. Ideally, medical management would try to balance the low resistance of the AVM by lowering the SVR; however, the diastolic flow reversal can result in diastolic hypotension and inadequate coronary artery perfusion. These management principles are in direct conflict with each other. Therefore, a balance is required in attempting to raise the diastolic BP to restore the coronary perfusion pressure and not further raising the SVR and inhibiting perfusion of the organs. Because of the compromised coronary perfusion, tachycardia and increasing myocardial VO2 should be avoided.
HF secondary to VGAM can be resistant to medical management and only improves after endovascular embolization of the malformation. Early endovascular intervention can be associated with higher rates of complications such as intracranial hemorrhage and death. This creates a clinical dilemma in the management of these infants. It would be ideal to medically manage the HF of these patients to achieve some postnatal growth and perform the endovascular treatment at a later time. However, with clinical deterioration, endovascular intervention needs to be undertaken prior to the occurrence of severe multiorgan failure.9 The establishment of a multidisciplinary team, including intensivist, anesthesiologist, cardiologist, neurosurgeon, radiologist among others, is important to identify those neonates with VGAM at high risk of complications secondary to HOHF where the risks of early embolization are outweighed by the risks of further efforts toward medical management.
References
Raybaud, C. A., Strother, C. M. & Hald, J. K. Aneurysms of the vein of Galen: embryonic considerations and anatomical features relating to the pathogenesis of the malformation. Neuroradiology 31, 109–128 (1989).
Houser, O. W., Campbell, J. K., Campbell, R. J. & Sundt, T. M. Jr. Arteriovenous malformation affecting the transverse dural venous sinus−an acquired lesion. Mayo Clin. Proc. 54, 651–661 (1979).
Duran, D. et al. Mutations in chromatin modifier and ephrin signaling genes in vein of Galen malformation. Neuron 101, 429.e4–443.e4 (2019).
Revencu, N. et al. Rasa1 mutations and associated phenotypes in 68 families with capillary malformation-arteriovenous malformation. Hum. Mutat. 34, 1632–1641 (2013).
Frawley, G. P., Dargaville, P. A., Mitchell, P. J., Tress, B. M. & Loughnan, P. Clinical course and medical management of neonates with severe cardiac failure related to vein of Galen malformation. Arch. Dis. Child. Fetal Neonatal Ed. 87, F144–F149 (2002).
Cumming, G. R. Circulation in neonates with intracranial arteriovenous fistula and cardiac failure. Am. J. Cardiol. 45, 1019–1024 (1980).
Cordova, E. G. et al. Vein of Galen malformation. Neoreviews 21, e678–e686 (2020).
Lasjaunias, P. L. et al. The management of vein of Galen aneurysmal malformations. Neurosurgery 59, S184–S194 (2006).
Patel, N., Mills, J. F., Cheung, M. M. & Loughnan, P. M. Systemic haemodynamics in infants with vein of Galen malformation: assessment and basis for therapy. J. Perinatol. 27, 460–463 (2007).
Chevret, L. et al. Severe cardiac failure in newborns with vgam. prognosis significance of hemodynamic parameters in neonates presenting with severe heart failure owing to vein of Galen arteriovenous malformation. Intensive Care Med. 28, 1126–1130 (2002).
Dahdah, N. S., Alesseh, H., Dahms, B. & Saker, F. Severe pulmonary hypertensive vascular disease in two newborns with aneurysmal vein of Galen. Pediatr. Cardiol. 22, 538–541 (2001).
Giorgi, L. et al. Management and outcomes of neonatal arteriovenous brain malformations with cardiac failure: a 17 years’ experience in a tertiary referral center. J. Pediatr. 218, 85.e2–91.e2 (2020).
Taffin, H. et al. Long-term outcome of vein of Galen malformation. Dev. Med. Child Neurol. 62, 729–734 (2020).
McElhinney, D. B., Halbach, V. V., Silverman, N. H., Dowd, C. F. & Hanley, F. L. Congenital cardiac anomalies with vein of Galen malformations in infants. Arch. Dis. Child. 78, 548–551 (1998).
Hansen, D. et al. Pediatric knowledge update: approach to the management of vein of Galen aneurysmal malformations in neonates. Surg. Neurol. Int. 7, S317–S321 (2016).
Fullerton, H. J., Aminoff, A. R., Ferriero, D. M., Gupta, N. & Dowd, C. F. Neurodevelopmental outcome after endovascular treatment of vein of Galen malformations. Neurology 61, 1386–1390 (2003).
Paladini, D. et al. Vein of Galen aneurysmal malformation (VGAM) in the fetus: retrospective analysis of perinatal prognostic indicators in a two-center series of 49 cases. Ultrasound Obstet. Gynecol. 50, 192–199 (2017).
Arko, L., Lambrych, M., Montaser, A., Zurakowski, D. & Orbach, D. B. Fetal and neonatal MRI predictors of aggressive early clinical course in vein of Galen malformation. Am. J. Neuroradiol. 41, 1105–1111 (2020).
Saliou, G. et al. Pseudofeeders on fetal magnetic resonance imaging predict outcome in vein of Galen malformations. Ann. Neurol. 81, 278–286 (2017).
Jhaveri, S., Berenstein, A., Srivastava, S., Shigematsu, T. & Geiger, M. K. High output cardiovascular physiology and outcomes in fetal diagnosis of vein of Galen malformation. Pediatr. Cardiol. 42, 1416–1424 (2021).
Kulik, T. J. Pulmonary blood flow and pulmonary hypertension: is the pulmonary circulation flowophobic or flowophilic? Pulm. Circ. 2, 327–339 (2012).
Kumar, S., Sud, N., Fonseca, F. V., Hou, Y. & Black, S. M. Shear stress stimulates nitric oxide signaling in pulmonary arterial endothelial cells via a reduction in catalase activity: role of protein kinase C delta. Am. J. Physiol. Lung Cell. Mol. Physiol. 298, L105–L116 (2010).
Ogasa, T. et al. Flow-mediated release of nitric oxide in isolated, perfused rabbit lungs. J. Appl. Physiol. 91, 363–370 (2001).
van Grondelle, A. et al. Altering hydrodynamic variables influences PGI2 production by isolated lungs and endothelial cells. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 57, 388–395 (1984).
Kluckow, M. & Evans, N. Superior vena cava flow in newborn infants: a novel marker of systemic blood flow. Arch. Dis. Child. Fetal Neonatal Ed. 82, F182–F187 (2000).
Vaksmann, G. et al. Evaluation of vein of Galen arteriovenous malformation in newborns by two dimensional ultrasound, pulsed and colour Doppler method. Eur. J. Pediatr. 148, 510–512 (1989).
Thankavel, P. P. & Ramaciotti, C. Early echocardiographic predictor of heart failure in cerebral arteriovenous malformations. Cardiol. Young 26, 1008–1012 (2016).
Ciricillo, S. F. et al. Serial ultrasonographic evaluation of neonatal vein of Galen malformations to assess the efficacy of interventional neuroradiological procedures. Neurosurgery 27, 544–548 (1990).
Heuchan, A. M. & Bhattacharyha, J. Superior vena cava flow and management of neonates with vein of Galen malformation. Arch. Dis. Child. Fetal Neonatal Ed. 97, F344–F347 (2012).
De Rosa, G. et al. Outcome of neonates with vein of galen malformation presenting with severe heart failure: a case series. Am. J. Perinatol. 36, 169–175 (2019).
Seri, I. Cardiovascular, renal, and endocrine actions of dopamine in neonates and children. J. Pediatr. 126, 333–344 (1995).
Seri, I. & Evans, J. Controversies in the diagnosis and management of hypotension in the newborn infant. Curr. Opin. Pediatr. 13, 116–123 (2001).
Subhedar, N. V. Treatment of hypotension in newborns. Semin. Neonatol. 8, 413–423 (2003).
Joynt, C. & Cheung, P. Y. Treating hypotension in preterm neonates with vasoactive medications. Front. Pediatr. 6, 86 (2018).
Bristow, M. R. et al. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N. Engl. J. Med. 307, 205–211 (1982).
Chatterjee, K. & De Marco, T. Role of nonglycosidic inotropic agents: indications, ethics, and limitations. Med. Clin. North Am. 87, 391–418 (2003).
Wehling, M. Specific, nongenomic actions of steroid hormones. Annu. Rev. Physiol. 59, 365–393 (1997).
Noori, S. & Seri, I. Neonatal blood pressure support: the use of inotropes, lusitropes, and other vasopressor agents. Clin. Perinatol. 39, 221–238 (2012).
Barrington, K. J., Finer, N. N. & Chan, W. K. A blind, randomized comparison of the circulatory effects of dopamine and epinephrine infusions in the newborn piglet during normoxia and hypoxia. Crit. Care Med. 23, 740–748 (1995).
Cheung, P. Y. & Barrington, K. J. The effects of dopamine and epinephrine on hemodynamics and oxygen metabolism in hypoxic anesthetized piglets. Crit. Care 5, 158–166 (2001).
Liet, J. M. et al. Dopamine effects on pulmonary artery pressure in hypotensive preterm infants with patent ductus arteriosus. J. Pediatr. 140, 373–375 (2002).
Hunt, R. W., Evans, N., Rieger, I. & Kluckow, M. Low superior vena cava flow and neurodevelopment at 3 years in very preterm infants. J. Pediatr. 145, 588–592 (2004).
Overgaard, C. B. & Dzavik, V. Inotropes and vasopressors: review of physiology and clinical use in cardiovascular disease. Circulation 118, 1047–1056 (2008).
Shaffner. Roger’s Textbook of Pediatric Intensive Care 5th edn 279–283 and 1175–1177 (Wolters Kluwer, 2016).
Robel-Tillig, E., Knupfer, M., Pulzer, F. & Vogtmann, C. Cardiovascular impact of dobutamine in neonates with myocardial dysfunction. Early Hum. Dev. 83, 307–312 (2007).
Martinez, A. M., Padbury, J. F. & Thio, S. Dobutamine pharmacokinetics and cardiovascular responses in critically ill neonates. Pediatrics 89, 47–51 (1992).
Ferrara, J. J. et al. Effects of dopamine and dobutamine on regional blood flow distribution in the neonatal piglet. Ann. Surg. 221, 531–540 (1995).
Ruffolo, R. R. Jr. The pharmacology of dobutamine. Am. J. Med. Sci. 294, 244–248 (1987).
Klitzner, T. S., Shapir, Y., Ravin, R. & Friedman, W. F. The biphasic effect of amrinone on tension development in newborn mammalian myocardium. Pediatr. Res. 27, 144–147 (1990).
Binah, O., Legato, M. J., Danilo, P. Jr. & Rosen, M. R. Developmental changes in the cardiac effects of amrinone in the dog. Circ. Res. 52, 747–752 (1983).
Artman, M., Kithas, P. A., Wike, J. S. & Strada, S. J. Inotropic responses change during postnatal maturation in rabbit. Am. J. Physiol. 255, H335–H342 (1988).
Akita, T., Joyner, R. W., Lu, C., Kumar, R. & Hartzell, H. C. Developmental changes in modulation of calcium currents of rabbit ventricular cells by phosphodiesterase inhibitors. Circulation 90, 469–478 (1994).
Bianchi, M. O., Cheung, P. Y., Phillipos, E., Aranha-Netto, A. & Joynt, C. The effect of milrinone on splanchnic and cerebral perfusion in infants with congenital heart disease prior to surgery: an observational study. Shock 44, 115–120 (2015).
Hoffman, T. M. et al. Prophylactic Intravenous Use of Milrinone after Cardiac Operation in Pediatrics (Primacorp) Study. Prophylactic intravenous use of milrinone after cardiac operation in pediatrics. Am. Heart J. 143, 15–21 (2002).
Monrad, E. S. et al. Effects of milrinone on coronary hemodynamics and myocardial energetics in patients with congestive heart failure. Circulation 71, 972–979 (1985).
Samiee-Zafarghandy, S. et al. Safety of milrinone use in neonatal intensive care units. Early Hum. Dev. 91, 31–35 (2015).
Giaccone, A. et al. Milrinone pharmacokinetics and pharmacodynamics in neonates with persistent pulmonary hypertension of the newborn. Am. J. Perinatol. 34, 749–758 (2017).
Treschan, T. A. & Peters, J. The vasopressin system: physiology and clinical strategies. Anesthesiology 105, 599–612 (2006).
Cowley, A. W. Jr. & Liard, J. F. Vasopressin and arterial pressure regulation. Special Lecture. Hypertension 11, I25–I32 (1988).
Tamaki, T. et al. Vasodilation induced by vasopressin V2 receptor stimulation in afferent arterioles. Kidney Int. 49, 722–729 (1996).
Walker, B. R., Haynes, J. Jr., Wang, H. L. & Voelkel, N. F. Vasopressin-induced pulmonary vasodilation in rats. Am. J. Physiol. 257, H415–H422 (1989).
Bidegain, M. et al. Vasopressin for refractory hypotension in extremely low birth weight infants. J. Pediatr. 157, 502–504 (2010).
Masarwa, R. et al. Role of vasopressin and terlipressin in refractory shock compared to conventional therapy in the neonatal and pediatric population: a systematic review, meta-analysis, and trial sequential analysis. Crit. Care 21, 1 (2017).
Rios, D. R., Moffett, B. S. & Kaiser, J. R. Trends in pharmacotherapy for neonatal hypotension. J. Pediatr. 165, 697.e1–701.e1 (2014).
Pellicer, A. et al. Cardiovascular support for low birth weight infants and cerebral hemodynamics: a randomized, blinded, clinical trial. Pediatrics 115, 1501–1512 (2005).
Weiss, S. L. et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr. Crit. Care Med. 21, e52–e106 (2020).
Moffett, B. S. & Price, J. F. National prescribing trends for heart failure medications in children. Congenit. Heart Dis. 10, 78–85 (2015).
Mebazaa, A. et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the survive randomized trial. JAMA 297, 1883–1891 (2007).
Mathew, R. et al. Milrinone as compared with dobutamine in the treatment of cardiogenic shock. N. Engl. J. Med. 385, 516–525 (2021).
Pirracchio, R. et al. The effectiveness of inodilators in reducing short term mortality among patient with severe cardiogenic shock: a propensity-based analysis. PLoS ONE 8, e71659 (2013).
Ashida, Y., Miyahara, H., Sawada, H., Mitani, Y. & Maruyama, K. Anesthetic management of a neonate with vein of Galen aneurysmal malformations and severe pulmonary hypertension. Paediatr. Anaesth. 15, 525–528 (2005).
Karam, O., da Cruz, E. & Rimensberger, P. C. VGAM induced high-flow congestive heart failure responsive to Pge1 infusion. Int. J. Cardiol. 132, e60–e62 (2009).
Fanconi, S., Burger, R., Ghelfi, D., Uehlinger, J. & Arbenz, U. Hemodynamic effects of sodium bicarbonate in critically ill neonates. Intensive Care Med. 19, 65–69 (1993).
McSweeney, N. et al. Management and outcome of vein of Galen malformation. Arch. Dis. Child. 95, 903–909 (2010).
Geibprasert, S., Krings, T., Armstrong, D., Terbrugge, K. G. & Raybaud, C. A. Predicting factors for the follow-up outcome and management decisions in vein of Galen aneurysmal malformations. Childs Nerv. Syst. 26, 35–46 (2010).
Funding
R. Savani holds the William Buchanan Chair in Pediatrics and L.C. is supported by NIH Grant 1R01NS102617. No financial support was received for the development of this review.
Author information
Authors and Affiliations
Contributions
M.J.C. and D.A. contributed to the concept of the paper, wrote the initial and revised drafts of this manuscript, and approved the final manuscript as submitted. R. Sillero, R. Savani, and L.C. contributed to the conceptualization of the paper, reviewed and revised the manuscript, and approved the final manuscript as submitted. P.D. and L.M. reviewed the manuscript, made corrections and edits, and approved the manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
R. Savani is on the Scientific Advisory Council of Mallinckrodt Pharmaceuticals, which had no role in the development of this review. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cory, M.J., Durand, P., Sillero, R. et al. Vein of Galen aneurysmal malformation: rationalizing medical management of neonatal heart failure. Pediatr Res 93, 39–48 (2023). https://doi.org/10.1038/s41390-022-02064-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02064-1
This article is cited by
-
Endovascular treatment of vein of Galen aneurysmal malformation: hospital-based case series in two tertiary centers
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2024)
-
Vein of Galen aneurysmal malformation: does size affect outcome?
Neuroradiology (2024)
-
Use of levosimendan in hemodynamic management of heart failure in two neonates with intracranial arteriovenous shunts: a case series
Italian Journal of Pediatrics (2023)