Etiology of intracranial stenosis in young patients: a high-resolution magnetic resonance imaging study
Introduction
Intracranial artery stenosis is highly prevalent in stroke patients of Asian, African, and Hispanic ancestry (1). Atherosclerosis is the widely accepted underlying etiology, especially in old patients. In patients at a young age, however, intracranial stenosis has varied causes, such as dissection, moyamoya disease, fibromuscular dystrophy, and vasculitis (2-4). Current imaging techniques, including digital subtraction angiography, CT angiography, and magnetic resonance angiography (MRA) cannot provide direct proof of the casual etiology, because different pathologies can result in similar patterns of intracranial lumen stenosis. The vascular pathophysiology of intracranial stenosis in young patients has been less well studied.
In the past decade, the technique of high-resolution magnetic resonance imaging (HRMRI) has been developed (5-8). Emerging studies have suggested that HRMRI is reliable for detecting intracranial artery wall lesions, consistent with histology findings (7-11). On HRMRI, eccentric stenosis is suggestive of atherosclerosis while concentric stenosis is suggestive of non-atherosclerotic diseases, although overlap may occasionally exist (3,8,12-14). In this study, using HRMRI, we sought to observe the vessel wall properties of intracranial stenosis in Chinese young patients and investigate the underlying etiology.
Methods
Participants
This is a retrospective study based on a prospectively established HRMRI database, approved by the ethics committee of Peking Union Medical College Hospital (15). Formal written consents were obtained from all the patients.
Consecutive young adult patients (from 18 to 45 years old) with transcranial Doppler (peak velocity >160 cm/s) or MRA suspected middle cerebral artery (MCA) stenosis were recruited (15). Patients were excluded if they had: (I) bilateral MCA stenosis or definite moyamoya disease, i.e., bilateral stenosis and/or occlusion of the intracranial internal carotid artery and its proximal branches, with development of collateral network (moyamoya vessels) (16); (II) extracranial carotid or vertebral artery stenosis; (III) cardiac embolic stroke; (IV) normal MRA and HRMRI findings; (V) poor image quality.
It is known that there is a sharp rise in the prevalence of traditional risk factors over the age of 35 years and “young stroke” increasingly resembles ‘old’ stroke (17). To address whether this “sharp risk factors rise” is meaningful for intracranial vessel pathophysiology, we further divided patients into two subgroups: patients >35 years old and patients ≤35 years old.
MR protocol
From January 2007 to June 2013, all patients underwent conventional T2 weighted imaging, diffusion weighted imaging, three-dimensional time-of-flight MRA and bilateral MCA HRMRI. The details of HRMRI protocol were described elsewhere (15). Briefly, HRMRI was composed of cross-section T2 and T1 weighted imaging, which were perpendicular to M1 segment of MCA, with slice thickness 2 mm and gap 0–0.2 mm. Acquisition resolution was 0.25 mm × 0.25 mm (T2WI) and 0.3 mm × 0.3 mm (T1WI). TR/TE =3,000/40 ms (T2WI), 600/12 ms (T1WI). Gd-DTPA enhancement (0.1 mmol/kg) was performed with T1WI sequence.
After July 2013, the technique of HRMRI was updated in our institution. The patients were imaged using a new 3.0 T scanner (GE Discovery MR750). The new HR-T1WI was obtained using a fast spin echo 3D technique (CUBE T1WI). The parameters were as follow: TR 565 ms, TE 16 ms, FOV 20 cm, matrix 320×256, ZIP 512, slice thickness 0.8 mm, ZIP 2. The display resolution were 0.4 mm × 0.4 mm × 0.4 mm. One hundred twenty coronal slices cover anterior and posterior circulation were acquired with scan time 5 min.
Image analysis
All MR images were transformed to a workstation for analysis. The eccentricity, degree of stenosis, and remodeling types of MCA lesions were analyzed by two experienced HRMRI reviewers (ML Li and YY Xu) blinded to the clinical data. The differences between two observers were solved by consensus.
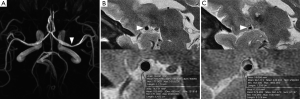
Eccentric stenosis were identified if the thinnest part of the wall was estimated to be less than 50% of the thickest point on at least one image slice of T2 weighted images (15). Concentric stenosis was diagnosed if the thinnest part of the wall was estimated to be no less than 50% of the thickest point on all image slices or a stenosis without wall thickening (8). We defined the reference sites as the nearest plaque-free segments proximal or distal to the maximal lumen narrowing sites. The degree of stenosis on HRMRI was calculated as follows: (1-luminal area at the maximal lumen narrowing site/reference luminal area) ×100% (15,18). The remodeling ratio of MCA stenosis was defined as the ratio of the outer-wall boundary area at the maximal lumen narrowing site to that at the reference site (19). Expansive remodeling was defined for a remodeling ratio of more than 1.05 (19). Constrictive remodeling was defined for a remodeling ratio of less than 0.95 (19) (Figure 1).
Statistical analysis
The intraclass correlation coefficient was used to find the intra-observer and inter-observer reproducibility for the measurements of luminal area and vessel wall area. Continuous variables were described as mean ± standard deviation in normally distributed data. The continuous variables between the two groups were compared by the independent samples t-test. Categorical variables were compared by the chi-square test or Fisher exact test. The variables were entered the binary Logistic regression model to study the relationship between predictive variables (atherosclerosis factors, eccentricity and remodeling ratio) and the dependent variable (cerebral infarction). A value of P<0.05 was used to indicate statistical significance.
Results
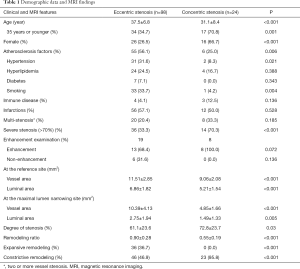
From Jan. 2009 to Aug. 2015, two hundred and forty-eight young patients with MCA stenosis were recruited. One hundred cases with moyamoya disease, 15 with bilateral MCA stenosis, 3 with cardiac embolic stroke, 12 with extra-cranial artery stenosis and 9 with poor image quality were excluded. The data of 122 patients with unilateral MCA stenosis were finally analyzed (Table 1). Of them, 68 patients were diagnosed with ischemic stroke, confirmed by diffusion weighted and/or T2 weighted imaging. Seven patients had systemic immune diseases. The remaining patients had non-specific symptoms such as headache and dizziness, etc., but no lesion was found on T2 weighted and diffusion weighted images. No patients with dissection or reversible cerebral vasoconstriction syndrome were clinically diagnosed. The intra-observer and inter-observer reproducibility of measurements of the luminal area were 0.882 (95% CI, 0.689–0.974) and 0.929 (95% CI, 0.667–0.983). The intra-observer and inter-observer reproducibility of measurements of the wall area were 0.848 (95% CI, 0.595–0.954) and 0.974 (95% CI, 0.950–0.990).
Full table
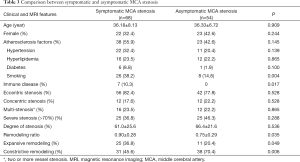
The demographic data and wall morphologic characteristics were summarized in Table 1. Eccentric stenosis was observed in 98 (80.3%) patients and concentric stenosis in 24 (19.7%) patients. The patients with eccentric stenosis were older (P<0.001), and more likely had atherosclerosis risk factors (P=0.016). The patients with concentric were more likely to be female (P<0.001) and 35 years old or younger (P=0.001). All concentric stenosis (100%) showed constrictive artery remodeling, while eccentric stenosis had heterogeneous remodeling types (P<0.001). In 27 patients with available enhanced images, six patients with eccentric stenosis had enhancement (13/19, 68.4%), as compared with concentric stenosis (8/8, 100%; P=0.072) (Figure 2).
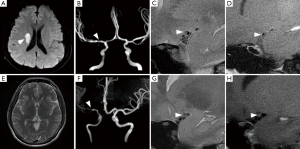
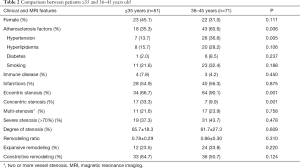
In subgroup analysis, there were 51 patients ≤35 years old and 71 patients >35 years old. Compared with the patients ≤35 years old, those aged 35 years old or older had much higher prevalence of atherosclerosis factors (P=0.006), and eccentric stenosis (P=0.001, Table 2). Most of patients with concentric stenosis were ≤35 years old (17/24, 70.8%) and were female (16/24, 66.7%).
Full table
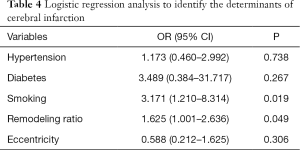
A binary Logistic regression model was constructed to study the relationship between cerebral infarction and other variables including atherosclerosis factors, eccentricity and remodeling ratio (details of univariate analysis are listed in Table 3 in supporting information). Binary Logistic analysis suggested smoking (OR =3.171; 95% CI, 1.210–8.314; P=0.019) and remodeling ratio (OR =1.625; 95% CI, 1.001–2.636; P=0.049) were independent predictive factors for symptomatic stenosis (Table 4).
Full table
Full table
Discussion
Evidence from the comparative studies between HRMRI and histology has supported that intracranial eccentric wall lesions represent the pathology of atherosclerosis (7,9-11). In this study, a high percentage (80.3%) of Chinese young patients with unilateral MCA stenosis had such eccentric wall lesions on HRMRI. Unsurprisingly, these patients were older and more likely to have atherosclerotic risk factors than those with concentric stenosis. Further analysis suggested that even in those ≤35 years old, eccentric stenosis was observed in as high as 2/3 of the patients. Taken together, our study suggests that atherosclerosis is the most common cause of intracranial stenosis in Chinese young patients, similar to the old (20,21). On the other hand, the patients aged 36 to 45 years old had more traditional vascular risk factors and eccentric stenosis than those ≤35 years old, supporting that the rising incidence of traditional vascular risk factors have effects on intracranial artery pathophysiology (17,21,22).
Although intracranial concentric wall lesions can be seen in patients with atherosclerosis, they were more frequently reported infective or non-infective vasculitis, and other vasculopathy (3,8,23,24). In this study, 19.7% patients had concentric wall lesions. Most of them were female, younger than 35 years old, and without atherosclerosis risk factors. High proportion of wall enhancement was observed, suggesting active inflammation and high intra-wall microvessel density (25). All the concentric stenosis showed unique constrictive remodeling, in comparison with the heterogeneous remodeling types in eccentric stenosis. Overall, the patients with concentric stenosis had different imaging and clinical features from those with eccentric wall lesions, i.e., presumed atherosclerosis. However, because the diagnostic tests including spinal tap tests and brain biopsies were not routinely performed, the stratified etiologies of concentric stenosis were undefined.
Our results are potentially clinically important. It has been known that the prognosis of young stroke is not as favorable as previously thought, with respect either to mortality, or cardiovascular disease, or to psychosocial consequences (17). Early etiology diagnosis and pathophysiology evaluation of intracranial stenosis are meaningful. For example, aggressive risk factors control, anti-thrombosis treatments and life style modification may be beneficial for premature intracranial atherosclerosis. HRMRI is helpful for giving a clue for further diagnostic procedures and establishing the treatment strategy.
There have been several studies on intracranial stenosis in young patients. In a multinational cohort study in Europe, intracranial atherosclerotic stenosis and occlusions (11.8%) were more prevalent than extracranial carotid artery disease (8.9%) in patients ≤55 years old with ischemic stroke and transient ischemic attacks (26). In another single-center cohort study in South Korea, using HRMRI, intracranial atherosclerosis was diagnosed in 27.4% patients aged ≤55 years old with unilateral MCA stenosis and with no or minimal (≤1) atherosclerotic risk factors (24). Comparatively, the strength of current study was that the patient recruitment was based on the presence of intracranial artery stenosis, regardless of clinical background (atherosclerosis risk factors and ischemic events). This inclusion criterion and the application of HRMRI made it possible to give an analysis to the underlying etiology of the vessel lesion patterns.
Unfortunately, current study suffered from several limitations. First, this was a single-center retrospective study in Chinese, a population with high prevalence of intracranial atherosclerosis. The ethnic bias couldn’t be excluded. Second, the follow-up imaging was not regularly performed. Some rare conditions including unilateral moyamoya disease may have been underestimated (27).
Conclusions
The current HRMRI study provides strong evidence that atherosclerosis is the most common cause of intracranial stenosis in Chinese young patients. Non-atherosclerosis disease is an important etiology in young female, especially in the patients aged 35 years old or younger.
Acknowledgements
Funding: This study is supported by Program for New Century Excellent Talents in University of China (NCET-12-0069), National Natural Science Foundation of China (81471207) and the Capital Health Research and Development of Special (2014-4-4015).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This is a retrospective study based on a prospectively established HRMRI database, approved by the ethics committee of Peking Union Medical College Hospital (No. JS-872). Formal written consents were obtained from all the patients.
References
- Gorelick PB, Wong KS, Bae HJ, et al. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke 2008;39:2396-9. [Crossref] [PubMed]
- Camilo O, Goldstein LB. Non-atherosclerotic vascular disease in the young. J Thromb Thrombolysis 2005;20:93-103. [Crossref] [PubMed]
- Mandell DM, Matouk CC, Farb RI, et al. Vessel wall MRI to differentiate between reversible cerebral vasoconstriction syndrome and central nervous system vasculitis: preliminary results. Stroke 2012;43:860-2. [Crossref] [PubMed]
- Siddiq F, Chaudhry SA, Vazquez G, et al. Intracranial stenosis in young patients: unique characteristics and risk factors. Neuroepidemiology 2012;38:148-53. [Crossref] [PubMed]
- Bodle JD, Feldmann E, Swartz RH, et al. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke 2013;44:287-92. [Crossref] [PubMed]
- Li ML, Xu WH, Song L, et al. Atherosclerosis of middle cerebral artery: evaluation with high-resolution MR imaging at 3T. Atherosclerosis 2009;204:447-52. [Crossref] [PubMed]
- Majidi S, Sein J, Watanabe M, et al. Intracranial-derived atherosclerosis assessment: an in vitro comparison between virtual histology by intravascular ultrasonography, 7T MRI, and histopathologic findings. AJNR Am J Neuroradiol 2013;34:2259-64. [Crossref] [PubMed]
- Swartz RH, Bhuta SS, Farb RI, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology 2009;72:627-34. [Crossref] [PubMed]
- Turan TN, Rumboldt Z, Granholm AC, et al. Intracranial atherosclerosis: correlation between in-vivo 3T high resolution MRI and pathology. Atherosclerosis 2014;237:460-3. [Crossref] [PubMed]
- Chen XY, Wong KS, Lam WW, et al. High signal on T1 sequence of magnetic resonance imaging confirmed to be intraplaque haemorrhage by histology in middle cerebral artery. Int J Stroke 2014;9:E19. [Crossref] [PubMed]
- van der Kolk AG, Zwanenburg JJ, Denswil NP, et al. Imaging the intracranial atherosclerotic vessel wall using 7T MRI: initial comparison with histopathology. AJNR Am J Neuroradiol 2015;36:694-701. [Crossref] [PubMed]
- Urowitz MB, Ibanez D, Gladman DD. Atherosclerotic vascular events in a single large lupus cohort: prevalence and risk factors. J Rheumatol 2007;34:70-5. [PubMed]
- Obusez EC, Hui F, Hajj-Ali RA, et al. High-resolution MRI vessel wall imaging: spatial and temporal patterns of reversible cerebral vasoconstriction syndrome and central nervous system vasculitis. AJNR Am J Neuroradiol 2014;35:1527-32. [Crossref] [PubMed]
- Yang WQ, Huang B, Liu XT, et al. Reproducibility of high-resolution MRI for the middle cerebral artery plaque at 3T. Eur J Radiol 2014;83:e49-55. [Crossref] [PubMed]
- Xu WH, Li ML, Gao S, et al. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis 2010;212:507-11. [Crossref] [PubMed]
- Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo) 2012;52:245-66. [Crossref] [PubMed]
- Maaijwee NA, Rutten-Jacobs LC, Schaapsmeerders P, et al. Ischaemic stroke in young adults: risk factors and long-term consequences. Nat Rev Neurol 2014;10:315-25. [Crossref] [PubMed]
- Varnava AM, Mills PG, Davies MJ. Relationship between coronary artery remodeling and plaque vulnerability. Circulation 2002;105:939-43. [Crossref] [PubMed]
- Schoenhagen P, Ziada KM, Kapadia SR, et al. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation 2000;101:598-603. [Crossref] [PubMed]
- Niu JW, Gao S, Cui LY, et al. Intracranial atherosclerosis in Chinese young adult stroke patients. J Stroke Cerebrovasc Dis 2014;23:1519-23. [Crossref] [PubMed]
- Wang Y, Zhao X, Liu L, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke 2014;45:663-9. [Crossref] [PubMed]
- Kissela BM, Khoury JC, Alwell K, et al. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology 2012;79:1781-7. [Crossref] [PubMed]
- Mossa-Basha M, Hwang WD, De Havenon A, et al. Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes. Stroke 2015;46:1567-73. [Crossref] [PubMed]
- Ahn SH, Lee J, Kim YJ, et al. Isolated MCA disease in patients without significant atherosclerotic risk factors: a high-resolution magnetic resonance imaging study. Stroke 2015;46:697-703. [Crossref] [PubMed]
- Wasserman BA. Advanced contrast-enhanced MRI for looking beyond the lumen to predict stroke: building a risk profile for carotid plaque. Stroke 2010;41:S12-6. [Crossref] [PubMed]
- von Sarnowski B, Schminke U, Tatlisumak T, et al. Prevalence of stenoses and occlusions of brain-supplying arteries in young stroke patients. Neurology 2013;80:1287-94. [Crossref] [PubMed]
- Park EK, Lee YH, Shim KW, et al. Natural history and progression factors of unilateral moyamoya disease in pediatric patients. Childs Nerv Syst 2011;27:1281-7. [Crossref] [PubMed]


