- Department of Neurology, UCLA Comprehensive Stroke Center, University of California, Los Angeles, Los Angeles, CA, United States
Background: Chronic subdural hematoma (cSDH) is a debilitating condition with a high rate of recurrence after surgical evacuation.
Summary: This review is focused on middle meningeal artery (MMA) embolization to treat cSDH. We discuss the underlying pathophysiology of chronic subdural hematoma and how cessation of arterial flow may resolve a venous hemorrhage. We also present the current evidence for MMA embolization and the roadmap for future trials.
Conclusion: Frequent multimodal imaging and cSDH sampling would enable us to understand mechanisms of MMA embolization in cSDH treatment and therefore improve our ability to offer MMA embolization to the eligible population.
Chronic Subdural Hematoma: Clinical Presentation and Treatment Options
Chronic subdural hematoma (cSDH) is a collection of blood, blood degradation products, and fluids encapsulated in the potential space between the arachnoid and the dura known as the subdural space. cSDH is relatively common and it has increased in frequency in parallel to an increase in the aging population. It is estimated to occur in 17–20 patients per 100,000 population per year (1, 2), which is twice the frequency of aneurysmal subarachnoid hemorrhage (3). It commonly presents with unspecific symptoms of cognitive or behavioral changes. Its insidious progression poses a diagnostic challenge that leads to its “chronic” discovery. A 6–12-months mortality of 30% (4) testifies to the high disease burden.
Subdural hematoma occurs spontaneously or as result of trauma. Use of antiplatelets or coagulopathy (pharmacologic or due to hepatic failure) increases the propensity for hemorrhage (5). Surgical treatment in cases of cSDH with significant mass effect (usually >10 mm blood thickness or >5 mm midline shift) is indicated and it is commonly performed through a single burr-hole or craniotomy drainage and irrigation (6). However, between 9.4 (7) and 30% (8) of cases will experience re-accumulation of hematoma. Among patients with a one-time SDH recurrence, a subsequent hematoma recurrence has been observed in nearly half (9). Factors increasing risk of recurrence include diabetes, liver dysfunction, use of anticoagulants, and post-operative residual air in the subdural space (10, 11).
Chronic Subdural Hematoma: Pathophysiology
There have been observational speculations about the pathophysiology of cSDH recurrence. The dominant theory revolves around rupture of bridging veins traversing from the brain to draining dural sinuses within the subdural space (12), but there are several characteristics of cSDH that argue for a more complex process: (1) cSDH takes several weeks to grow (13) that is longer than expected from a venous source of bleeding; (2) cSDH often extends across the cerebral convexities away from medial draining sinuses where bridging veins are predominantly located; and (3) acute hemorrhage is only observed in 9% of patients with growing cSDH (14), suggesting acute hemorrhage is not the etiology for a majority of cases. Alternative explanations have centered on a self-propagating cycle of inflammation, angiogenesis, exudation, and hemorrhage, which is described below.
cSDH occurs in a potential space between the brain and the dura populated with “dural border cells” (15). Initial hemorrhage occurs within the subdural space following a minor trauma in the context of increased traction from a shrinking aging brain. Hemorrhage leads to proliferation of dural border cells (16). In 21% of cases with acute SDH a sustained state of inflammation ensues leading to evolution of cSDH (17): influx of inflammatory cells to the injured dural border cells layer promotes proliferation of the cells into forming new membranes. Disruption of dural border cell layer leads to deposition of collagenous material to form the fibro-cellular connective tissue (18) in a process mirroring wound repair. Disrupted dural border cell layer subsequently reorganizes into the outer and the inner membranes, which are adjacent to dura and arachnoid layers, respectively (19). The inner membrane is a fibro-collagenous tissue with minimal vasculature or inflammation that does not contribute to cSDH growth (19), but in contrast, the outer membrane has been populated with neutrophils, lymphocytes, macrophages, eosinophils, and newly-formed vessels (20). Some studies have associated the angiographic “wispiness” of distal MMA branches with neovascularization in this layer (21). The new blood vessels have thin-walls with thin or no basement membrane and are devoid of smooth muscle cells or pericytes (20, 22) allowing continuous exudation of plasma and RBC into the subdural space (19, 22). Fragility of blood vessels in the outer membrane has been associated with intermittent acute bleeding in cSDH manifested as CT hyperdense foci (20).
Secretion of pro-inflammatory factors, such as vascular endothelial-derived growth factor, tissue plasminogen activator, angiopoietin-2, matrix metalloproteinases, tumor necrosis factor-α, interleukin (IL)-6, IL-8, thrombomodulin, and basic fibroblast growth factor (23), by the outer membrane into the subdural space fuels ongoing inflammation in a contained collection of blood, blood degradation products, and exudated fluids. The relevant question is how occlusion of middle meningeal artery (MMA) leads to the resolution of a self-perpetuating contained sac of inflammation in the subdural space. Figure 1 summarizes the factors contributing to the formation of cSDH.
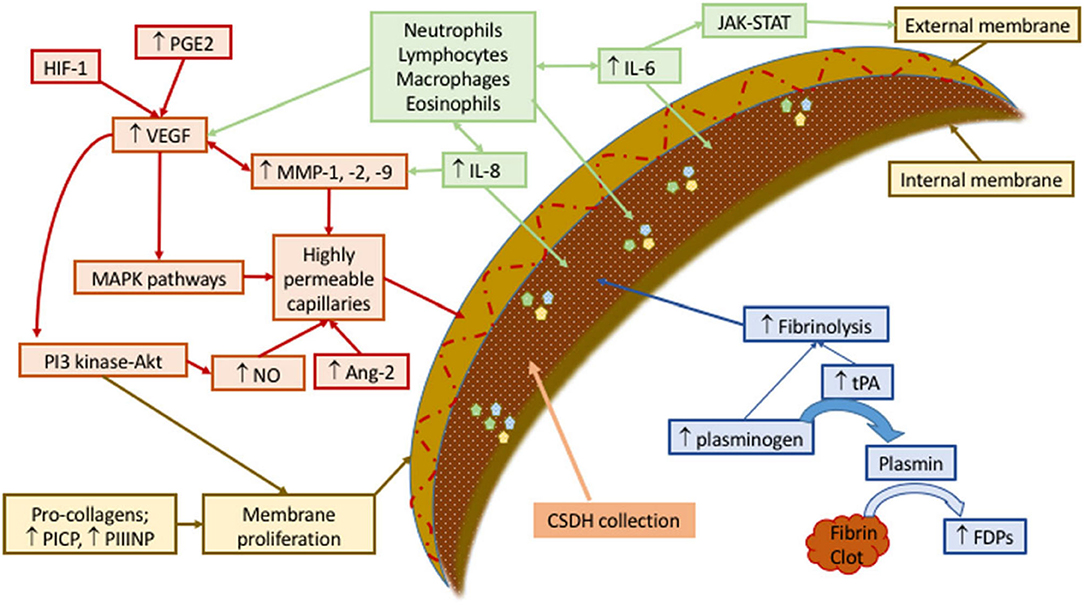
Figure 1. Schematic representation of mechanisms involved in development and sustenance of cSDH. Contributing factors have been labeled as green (recruiting inflammatory cell), red (forming permeable capillaries), brown (forming inner and outer membranes), and blue (ongoing hemorrhage due to fibrinolysis). Ang, angiopoietin; FDPs, fibrin/fibrinogen degradation products; HIF, hypoxia-inducible factor; IL, interleukin; JAK-STAT, Janus kinase-signal transducer and activator of transcription; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; NO, nitric oxide; PGE, prostaglandin E; PI3-Akt, phosphatidylinositol 3-kinase-serine/threonine kinase; PICP, procollagen type 1; PIIINP, procollagen type 3; tPA, tissue plasminogen activator; VEGF, vascular endothelial growth factor. Reproduced from (24) under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
Middle Meningeal Artery Embolization as a Treatment Option for cSDH
cSDH recurrence is not uncommon. Surgical drainage of cSDH fails to cure in 9.4–30% (7) of patients. Some undergo repeat surgical treatment but if surgical treatment fails once, further recurrences are more common and some estimate the rate to be as high as 46% (9). Remaining options are limited and include peritoneal shunt, application of Ommaya reservoir, or endoscopic drainage and debridement (6, 10), none of which are proven to be effective.
Komiyama first introduced MMA embolization as a treatment option for recurrent cSDH in 1994 (25). Several case series have then been published on utilization of MMA embolization to treat cSDH refractory to surgical drainage. In a review, 21 cases of cSDH failed 1–7 times surgical drainage were successfully treated with MMA embolization (9). In a larger report of 72 consecutive patients (26) MMA embolization was performed in cSDH as a sole therapy or an adjunct treatment prior to surgical drainage in 27 and 45 patients, respectively. The success rate for MMA treatment was remarkable: There was no failure for MMA embolization as the sole treatment, and a 2.2% failure rate was reported when MMA occlusion was combined with surgical treatment. A comparison with historical control patients treated only with surgery revealed MMA embolization outperformed surgical drainage (p < 0.001).
MMA embolization has also been the subject of case-controlled studies. In a meta-analysis of 8 case-control studies (27), the rate of treatment failure in MMA embolization and conventional surgical treatment was 2.1 vs. 27.7%, respectively (OR 0.87, 95% CI 0.026–0.292, p < 0.001). In other more recent systematic reviews, the failure for cSDH patients undergoing MMA embolization was 4.1 and 2.4% for primary and recurrent cases, respectively (28). The need for surgical rescue in patient undergoing MMA embolization was 2.7% (29). MMA embolization had a 26 and 20% risk reduction for cSDH recurrence and surgical intervention, respectively (29). The rate of complications for MMA embolization was 1.2% (29). Given those promising results, a randomized clinical trial (ChiCTR1800018714), three parallel assignment open label studies (NCT04065113, NCT04095819, NCT04270955) and a single arm open label study (NCT03307395) are recruiting patients to further study MMA embolization in cSDH. In addition, they are more clinical studies planned but not yet commenced (NCT04272996, EMBOLISE NCT04402632, and EMPROTECT NCT04372147).
How Does Blocking Arterial Blood Flow Cure Subdural Hematoma?
MMA embolization has proven effective in treating cSDH in non-randomized case-control studies. However, it is important to understand the mechanism for cessation of arterial blood supply to treat a hemorrhage that is venous in nature. Understanding mechanisms of therapeutic effects will enable us to offer MMA embolization to the eligible population and improve design of future randomized trials to provide high quality evidence for effectiveness of MMA embolization to treat cSDH.
MMA is a branch of the maxillary artery, which itself is derived from the external carotid artery. It enters the skull through the foramen spinosum, courses through dura and divides into frontal and parietal branches (Figure 2). The MMA, together with anterior meningeal artery and posterior meningeal artery, supplies the meninges. Therefore, the MMA supplies blood to cSDH located in the mid-anterior to mid-posterior cerebral convexity.
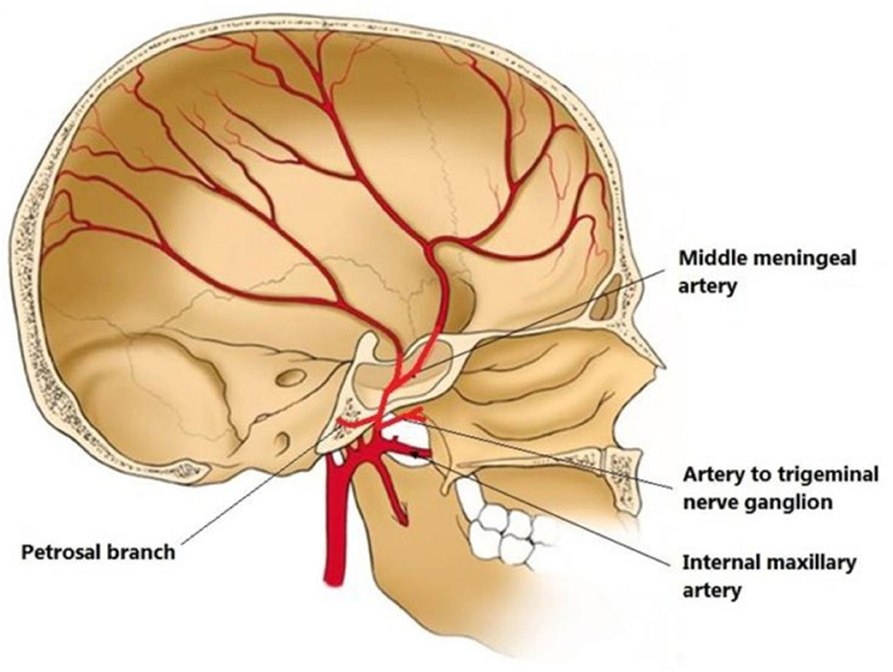
Figure 2. Illustration of middle meningeal artery (MMA) anatomy originated from internal maxillary artery and coursing in the inner skull. From (30) distributed under Creative Commons Public Domain Mark 1.0.
It is important to mention anastomoses of distal MMA branches with ophthalmic artery via the recurrent meningeal artery, and with posterior auricular artery supplying facial nerve, since inadvertent leaking of embolizing particle materials can cause ophthalmic nerve and facial nerve injuries, respectively (31). An ophthalmic artery origin of MMA has been observed in 13.8% of patients with cSDH compared with 0.7% of patients with epistaxis selected as control (32). Whether it is causative or adaptive in recurrent cSDH, or simply a co-occurrence, an ophthalmic origin of MMA prohibits embolization as a treatment for cSDH. Another technical consideration is the choice of embolizing material: majority of studies have utilized polyvinyl alcohol (PVA) particles (14) and few have used liquid embolic material. There are theoretical advantages for particles or liquid materials: particles have more distal penetrance and block most distal branches that may receive collateral perfusion from other arteries than the MMA, however, particles are not opaque and they are hard to visually track. On the other hand, small size of microcatheters only allows limited volume delivery of particles, while larger amounts of liquid embolic agents can be injected under constant pressure. Clinical studies are warranted to compare efficacy of different embolizing materials in treating cSDH.
Utilization of the MMA in treating cSDH has also been suggested by the observation that MMA appears engorged in cSDH (33). As reviewed above, collection of cSDH is caused by an active biologic process of exudation and formation of loose vasculature prone to spontaneous bleeding. Therefore, occlusion of the MMA does not simply block blood pumping into the subdural space, but rather affects the complex biology of inner and outer membranes lining the cSDH cavity. cSDH CT images obtained after MMA embolization have shown contrast material enhancement of dura, capsular membrane, septations, and subdural hematoma fluid, suggesting a continuous vasculature between the cSDH membranes and the MMA (34). It is therefore expected that MMA embolization causes ischemia in the outer membrane, as well as the inner membrane, which leads to resolution of cSDH.
In order to understand the pathophysiology of cSDH resolution following MMA embolization it is helpful to discuss a similar pathology treated with arterial embolization: hypervascular intracranial tumors and their pre-surgical embolization. Arterial embolization is used as an adjunct to surgical resection to diminish intraoperative hemorrhage and decrease tumor size. It is typically used in hypervascular tumors, such as meningioma, located in deep cranial locations like skull base (35). Histopathologic assessment of embolized tumors have revealed that arterial embolization induces cellular dissociation and ischemic cellular changes such as cell shrinkage, nuclear pyknosis, and karyorrhexis (36). There are areas of confluent necrosis and micronecrosis (37), as well as apoptosis in the perinecrotic areas (37). Polymorphonuclear infiltration follows and leads to the formation of perivascular cuffing and inflammatory reaction in the surrounding area (38). Some degrees of cell proliferation and neo-angiogenesis follow inflammation (37). Super selective embolization of tumor feeding artery has been associated with 3.2% (38) to 5.1% (39) rates of tumor hemorrhage.
In contrast to pre-surgical tumor embolization, tissue specimens are not readily available in cSDH after MMA embolization. It has been suggested, however, that occlusion of MMA leads to ischemia in inner and outer membranes that subsequently impairs their biological role in sustaining cSDH. Beyond this speculation we do not know the details of biochemical cascades in cSDH and surrounding membranes following MMA embolization. We expect that a higher metabolic state of surrounding membranes makes them susceptible to ischemia and allows MMA embolization to selectively eliminate inner and outer membranes following necrosis and apoptosis. Cell death inevitably causes inflammation, cell proliferation, and neovascularization, but these processes just as in tumor embolization (38) do not usually cause swelling and hemorrhage in the cSDH collection sac (26, 27, 40, 41). In cSDH membranes surviving MMA embolization, ischemia probably impairs active processes of cell proliferation, angiogenesis, and secretion. These would halt secretion into the cSDH and allows the resorption to supersede and resolve cSDH. All of the above explanations are speculative but there are aspects of trial design that can help in validating probable processes of cSDH resolution.
How Can Clinical Trial Design Inform MMA Embolization?
Repeated brain MRI allows for tracking changes in the thickness and composition of surrounding membrane, as well as the inner sac composition and size following MMA embolization. Relative changes in inner vs. the more vascular outer membrane will inform on their susceptibility to ischemia. Contrast-enhanced MRI allows for evaluation of blood-brain barrier integrity that could be compromised by inflammation or ischemia. Monitoring the possible enhancement of surrounding membranes informs us of the baseline permeability within these membranes and the changes following MMA embolization. This approach has been implemented and a higher enhancement of cSDH membranes have been correlated with a shorter interval for hematoma recurrence (42). Positron emission tomography (PET) has been previously utilized in cSDH and fluorodeoxyglucose uptake attributed to high metabolic activity (43). The use of PET in trials may help to track metabolic activity following cessation of MMA blood flow. Those patients with a subdural shunt or Ommaya reservoir can also provide us with a valuable opportunity to sample the cSDH content before and after embolization to analyze levels of inflammatory factors (e.g., vascular endothelial-derived growth factor, tissue plasminogen activator, angiopoietin-2, matrix metalloproteinases, tumor necrosis factor-α, IL-6, IL-8, thrombomodulin, and basic fibroblast growth factor) and blood degradation products and thereby record the sequence of events following MMA embolization. The current studies on MMA embolization and cSDH have not reported such information (26, 27, 40, 41) and future studies may help to shed light on the pathophysiology of MMA embolization in cSDH resolution.
Patients with cSDH are either (1) surgically naive, (2) surgically failed, or (3) receiving MMA as adjunctive post-surgical modality. It is also important to choose a homogenous population for future trials since each of those sub-groups of patients have different propensities to fail treatment and re-accumulate hematoma. Progression or reduction of hematoma measured at certain time point has been commonly used as the primary outcome, but in a patient-centered approach it is important to include parameters such as time to restart antiplatelets or anticoagulants indicated due to other cerebrovascular or cardiovascular conditions. A patient's clinical response to treatment should be independently assessed as a trial endpoint. Since cSDH does not often cause focal neurologic symptoms, neurocognitive assessments may be used to track patients' clinical improvement. Imaging endpoints may include cSDH size, change in cSDH, or change in the degree of membrane enhancement following MMA embolization.
Given heterogeneity of the studied population, as well as differences in imaging modalities and measurement techniques, development of standardized methods of patient selection, and imaging analyses is recommended, to facilitate sample size estimation and statistical meta-analysis. There is a need for unbiased non-industry funded trials to impartially assess effectiveness and elucidate underlying mechanisms for MMA embolization in treating cSDH.
Conclusion
MMA embolization has been very effective in treating cSDH, but limited understanding of cSDH cure mechanisms curtails our ability to offer MMA embolization to the eligible population and improve the design of future randomized trials. By introducing frequent multimodal imaging and use of contrasted studies, as well as cSDH sampling, we may be able to monitor changes following MMA embolization and provide high quality evidence for the effectiveness of MMA embolization.
Author Contributions
PM and DL: conception, design, analysis, interpretation of data, critically revising the article, reviewed submitted version of manuscript, and administrative/technical/material support. PM: acquisition of data and drafting the article. DL: approved the final version of the manuscript on behalf of all authors and study supervision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
DL is consultant to Cerenovus, Genentech, Stryker, and Medtronic as Imaging Core Lab.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
cSDH, Chronic subdural hematoma; IL, interleukin; MMA, middle meningeal artery.
References
1. Balser D, Farooq S, Mehmood T, Reyes M, Samadani U. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J Neurosurg. (2015) 123:1209–15. doi: 10.3171/2014.9.JNS141550
2. Rauhala M, Luoto TM, Huhtala H, Iverson GL, Niskakangas T, Ohman J, Helen P. The incidence of chronic subdural hematomas from 1990 to 2015 in a defined Finnish population. J Neurosurg. (2019) 1–11. doi: 10.3171/2018.12.JNS183035
3. Etminan N, Chang HS, Hackenberg K, de Rooij NK, Vergouwen MDI, Rinkel GJE, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol. (2019) 76:588–97. doi: 10.1001/jamaneurol.2019.0006
4. Dumont TM, Rughani AI, Goeckes T, Tranmer BI. Chronic subdural hematoma: a sentinel health event. World Neurosurg. (2013) 80:889–92. doi: 10.1016/j.wneu.2012.06.026
5. Abe Y, Maruyama K, Yokoya S, Noguchi A, Sato E, Nagane M, et al. Outcomes of chronic subdural hematoma with preexisting comorbidities causing disturbed consciousness. J Neurosurg. (2017) 126:1042–6. doi: 10.3171/2016.3.JNS152957
6. Shapey J, Glancz LJ, Brennan PM. Chronic subdural haematoma in the elderly: is it time for a new paradigm in management? Curr Geriatr Rep. (2016) 5:71–7. doi: 10.1007/s13670-016-0166-9
7. Ko BS, Lee JK, Seo BR, Moon SJ, Kim JH, Kim SH. Clinical analysis of risk factors related to recurrent chronic subdural hematoma. J Korean Neurosurg Soc. (2008) 43:11–5. doi: 10.3340/jkns.2008.43.1.11
8. Nakaguchi H, Tanishima T, Yoshimasu N. Relationship between drainage catheter location and postoperative recurrence of chronic subdural hematoma after burr-hole irrigation and closed-system drainage. J Neurosurg. (2000) 93:791–5. doi: 10.3171/jns.2000.93.5.0791
9. Tempaku A, Yamauchi S, Ikeda H, Tsubota N, Furukawa H, Maeda D, et al. Usefulness of interventional embolization of the middle meningeal artery for recurrent chronic subdural hematoma: five cases and a review of the literature. Interv Neuroradiol. (2015) 21:366–71. doi: 10.1177/1591019915583224
10. Matsumoto K, Akagi K, Abekura M, Ryujin H, Ohkawa M, Iwasa N, et al. Recurrence factors for chronic subdural hematomas after burr-hole craniostomy and closed system drainage. Neurol Res. (1999) 21:277–80. doi: 10.1080/01616412.1999.11740931
11. Shiomi N, Sasajima H, Mineura K. Relationship of postoperative residual air and recurrence in chronic subdural hematoma. No Shinkei Geka. (2001) 29:39–44.
12. Ommaya AK, Yarnell P. Subdural haematoma after whiplash injury. Lancet. (1969) 2:237–9. doi: 10.1016/S0140-6736(69)90005-1
13. Gelabert-Gonzalez MM. Iglesias-Pais A. Garcia-Allut, and R. Martinez-Rumbo, Chronic subdural haematoma: surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. (2005) 107:223–9. doi: 10.1016/j.clineuro.2004.09.015
14. Fiorella D, Arthur AS. Middle meningeal artery embolization for the management of chronic subdural hematoma. J Neurointerv Surg. (2019) 11:912–5. doi: 10.1136/neurintsurg-2019-014730
15. Kolias AG, Chari A, Santarius T, Hutchinson PJ. Chronic subdural haematoma: modern management and emerging therapies. Nat Rev Neurol. (2014) 10:570–8. doi: 10.1038/nrneurol.2014.163
16. Inglis K. Subdural haemorrhage, cysts and false membranes; illustrating the influence of intrinsic factors in disease when development of the body is normal. Brain. (1946) 69:157–94. doi: 10.1093/brain/69.3.157
17. Izumihara A, Yamashita K, Murakami T. Acute subdural hematoma requiring surgery in the subacute or chronic stage. Neurol Med Chir. (2013) 53:323–8. doi: 10.2176/nmc.53.323
18. Heula AL, Sajanti J, Majamaa K. Procollagen propeptides in chronic subdural hematoma reveal sustained dural collagen synthesis after head injury. J Neurol. (2009) 256:66–71. doi: 10.1007/s00415-009-0048-6
19. Sato S, Suzuki J. Ultrastructural observations of the capsule of chronic subdural hematoma in various clinical stages. J Neurosurg. (1975) 43:569–78. doi: 10.3171/jns.1975.43.5.0569
20. Moskala M, Goscinski I, Kaluza J, Polak J, Krupa M, Adamek D, et al. Morphological aspects of the traumatic chronic subdural hematoma capsule: SEM studies. Microsc Microanal. (2007) 13:211–9. doi: 10.1017/S1431927607070286
21. Link TW, Rapoport BI, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: endovascular technique and radiographic findings. Interv Neuroradiol. (2018) 24:455–62. doi: 10.1177/1591019918769336
22. Yamashima T, Yamamoto S, Friede RL. The role of endothelial gap junctions in the enlargement of chronic subdural hematomas. J Neurosurg. (1983) 59:298–303. doi: 10.3171/jns.1983.59.2.0298
23. Pripp AH, Stanisic M. The correlation between pro- and anti-inflammatory cytokines in chronic subdural hematoma patients assessed with factor analysis. PLoS ONE. (2014) 9:e90149. doi: 10.1371/journal.pone.0090149
24. Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KLH, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflam. (2017) 14:108. doi: 10.1186/s12974-017-0881-y
25. Komiyama M, Yasui T, Tamura K, Nagata Y, Fu Y, Yagura H. Chronic subdural hematoma associated with middle meningeal arteriovenous fistula treated by a combination of embolization and burr hole drainage. Surg Neurol. (1994) 42:316–9. doi: 10.1016/0090-3019(94)90400-6
26. Ban SP, Hwang G, Byoun HS, Kim T, Lee SU, Bang JS, et al. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. (2018) 286:992–9. doi: 10.1148/radiol.2017170053
27. Srivatsan A, Srinivasan VM, Thomas A, Burkhardt JK, Johnson JN, Kan P. Perspective on safety and effectiveness of middle meningeal artery embolization for chronic subdural hematoma. World Neurosurg. (2019) 127:97–8. doi: 10.1016/j.wneu.2019.03.210
28. Haldrup M, Ketharanathan B, Debrabant B, Schwartz OS, Mikkelsen R, Fugleholm K, et al. Embolization of the middle meningeal artery in patients with chronic subdural hematoma-a systematic review and meta-analysis. Acta Neurochir. (2020) 162:777–84. doi: 10.1007/s00701-020-04266-0
29. Jumah F, Osama M, Islim AI, Jumah A, Patra DP, Kosty J, et al. Efficacy and safety of middle meningeal artery embolization in the management of refractory or chronic subdural hematomas: a systematic review and meta-analysis. Acta Neurochir. (2020) 162:499–507. doi: 10.1007/s00701-019-04161-3
31. Schirmer CM, Siddiqui AH. Commentary: middle meningeal artery embolization for chronic subdural hematoma: a series of 60 cases. Neurosurgery. (2019) 85: E1004–5. doi: 10.1093/neuros/nyy587
32. Fantoni M, Eliezer M, Serrano F, Civelli V, Labeyrie MA, Saint-Maurice JP, et al. High frequency of ophthalmic origin of the middle meningeal artery in chronic subdural hematoma. Neuroradiology. (2020) 62:639–44. doi: 10.1007/s00234-020-02363-6
33. Takizawa K, Sorimachi T, Ishizaka H, Osada T, Srivatanakul K, Momose H, et al. Enlargement of the middle meningeal artery on MR angiography in chronic subdural hematoma. J Neurosurg. (2016) 124:1679–83. doi: 10.3171/2015.5.JNS1567
34. Mureb MC, Kondziolka D, Shapiro M, Raz E, Haynes J, Farkas J, et al. DynaCT enhancement of subdural membranes after MMA embolization: insights into pathophysiology. World Neurosurg. (2020) 139:e265–70. doi: 10.1016/j.wneu.2020.03.188
35. Perry A, Chicoine MR, Filiput E, Miller JP, Cross DT. Clinicopathologic assessment and grading of embolized meningiomas: a correlative study of 64 patients. Cancer. (2001) 92:701–11. doi: 10.1002/1097-0142(20010801)92:3<701::AID-CNCR1373>3.0.CO;2-7
36. Jimenez-Heffernan JA, Corbacho C, Canizal JM, Perez-Campos A, Vicandi B, Lopez-Ibor L, et al. Cytological changes induced by embolization in meningiomas. Cytopathology. (2012) 23:57–60. doi: 10.1111/j.1365-2303.2010.00836.x
37. Barresi V, Branca G, Granata F, Alafaci C, Caffo M, Tuccari G. Embolized meningiomas: risk of overgrading and neo-angiogenesis. J Neurooncol. (2013) 113:207–19. doi: 10.1007/s11060-013-1117-3
38. Ng HK, Poon WS, Goh K, Chan MS. Histopathology of post-embolized meningiomas. Am J Surg Pathol. (1996) 20:1224–30. doi: 10.1097/00000478-199610000-00008
39. Carli DF, Sluzewski M, Beute GN, van Rooij WJ. Complications of particle embolization of meningiomas: frequency, risk factors, and outcome. AJNR Am J Neuroradiol. (2010) 31:152–4. doi: 10.3174/ajnr.A1754
40. Jang KM, Kwon JT, Hwang SN, Park YS, Nam TK. Comparison of the outcomes and recurrence with three surgical techniques for chronic subdural hematoma: single, double burr hole, and double burr hole drainage with irrigation. Korean J Neurotrauma. (2015) 11:75–80. doi: 10.13004/kjnt.2015.11.2.75
41. Kim E. Embolization therapy for refractory hemorrhage in patients with chronic subdural hematomas. World Neurosurg. (2017) 101:520–7. doi: 10.1016/j.wneu.2017.02.070
42. Nakagawa I, Park HS, Kotsugi M, Wada T, Takeshima Y, Matsuda R, et al. Enhanced hematoma membrane on DynaCT images during middle meningeal artery embolization for persistently recurrent chronic subdural hematoma. World Neurosurg. (2019) 126: e473–9. doi: 10.1016/j.wneu.2019.02.074
43. Nishida Y, Kobayashi E, Kubota D, Setsu N, Ogura K, Tanzawa Y, et al. Chronic expanding hematoma with a significantly high fluorodeoxyglucose uptake on (1)(8)F-fluorodeoxyglucose positron emission tomography, mimicking a malignant soft tissue tumor: a case report. J Med Case Rep. (2014) 8:349. doi: 10.1186/1752-1947-8-349
Keywords: endovascular treatment, embolization, interventional neuroradiology, chronic subdural hematoma, middle meningeal artery
Citation: Moshayedi P and Liebeskind DS (2020) Middle Meningeal Artery Embolization in Chronic Subdural Hematoma: Implications of Pathophysiology in Trial Design. Front. Neurol. 11:923. doi: 10.3389/fneur.2020.00923
Received: 28 April 2020; Accepted: 17 July 2020;
Published: 27 August 2020.
Edited by:
Osama O. Zaidat, Northeast Ohio Medical University, United StatesReviewed by:
Ameer E. Hassan, UTRGV School of Medicine, United StatesAshutosh Jadhav, University of Pittsburgh, United States
Farhan Siddiq, University of Missouri System, United States
Copyright © 2020 Moshayedi and Liebeskind. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David S. Liebeskind, dliebeskind@mednet.ucla.edu
 Pouria Moshayedi
Pouria Moshayedi David S. Liebeskind*
David S. Liebeskind*