Article Text
Abstract
Background Despite significant advancements in the procedural efficacy of mechanical thrombectomy in patients with ischemic stroke in recent years, there still remains a portion of the population that does not achieve good recanalization. The reasons for this may be varied. We hypothesized that static friction between the clot and the vessel, or catheter wall might contribute to the difficulty in removing the clot.
Objective To determine if there is a relationship between clot composition and the resistance to sliding (friction) which might contribute to resistance to clot removal.
Methods As clot composition can vary significantly, we investigated five different types of clot in order to measure their respective frictional properties. To do this, a custom-made testing apparatus was created, consisting of various replaceable low-friction surfaces on which the clots could be placed. The surface was then gradually tilted until the clots began to slide; the angle at which this occurred is related to the coefficient of friction of the clots. The experiment was repeated on a bovine aortic surface in order to confirm the results.
Results We found that fibrin-rich clots (<20% red blood cell content) have a significantly higher coefficient of friction than clots with a red blood cell content >20%. This result was confirmed by repeating the experiment on a bovine aortic surface as a representation of the interaction between clots and the arterial wall.
Conclusions The friction properties of clots were found to be related to the content ratio of fibrin to red blood cells. Future imaging techniques that could show fibrin and red blood cell content might help us to predict the ‘stickiness’ of a clot.
- Stroke
- Thrombectomy
Statistics from Altmetric.com
Introduction
Recent studies have shown that stentriever thrombectomy is an effective treatment for clot removal in patients with stroke.1–4 Despite significant advancements in procedural efficacy in recent years, there still remain, on average, approximately 25% of patients who do not achieve Thrombolysis in Cerebral Infarction 2b or greater recanalization.1 ,3–8 Studies have shown that lower recanalization rates, or a higher number of retrieval attempts, are associated with fibrin-rich clots.9–11 The reason for this is unknown. However Yuki et al10 hypothesize that the resistance of fibrin-rich clots to thrombectomy may be related to their mechanical properties; they are stiffer/harder than red blood cell (RBC)-rich clots, which results in a different interaction with the thrombectomy device.
During the thrombectomy procedure a clot is typically dislodged from its occlusive location and retrieved proximally. The clot will therefore experience sliding contact along the inside of a vessel and/or through the inside of a catheter. We propose that the resistance to sliding of the clot may contribute to the difficulty of removal via mechanical thrombectomy.
Clot composition can vary substantially from patient to patient. Several studies analyzing clots retrieved from patients have shown that the ratio of fibrin to RBC content can vary from 100% fibrin to almost 80% RBCs.12–14 In our laboratory, we have created a variety of blood clots of different compositions (from 0% to 80% RBC content) based on strict protocols, previously described by our group.15 Some mechanical characterization of clots has been investigated in the past, including tensile, compressive, and rheological properties.16 ,17 However, the frictional properties of clot have not yet been characterized.
In this study, we designed an experiment to determine if there is a relationship between clot composition and the resistance to sliding (friction) which might contribute to resistance to clot removal.
Methods and materials
Preparation of clots
Venous blood was obtained from sheep in order to prepare the clot samples for testing following the methodology previously described by our group.15 Sheep blood was chosen because it has been shown to be a suitable substitute for human blood for coagulation studies by Siller-Matula et al18 and the corresponding blood clots have been shown to be histologically similar to human blood and clots.15 Briefly, blood was collected (Ash Stream vets, Co. Mayo, Ireland) and anticoagulated with 3.2% sodium citrate solution (Sigma Aldrich, Ireland). It was then transported to the laboratory where it was stored at 4°C and blood was separated into plasma, buffy layer, and erythrocyte-rich layers using a centrifuge set at 550g for 15 min (Centurion Scientific, C2 series). The plasma and erythrocytes were harvested separately and then recombined in controlled ratios to give five different test groups of blood clots with varying composition: 0%, 5%, 20%, 40%, and 80% RBCs, respectively. These percentages represent the ratios in which the clots were prepared (ie, the volume of erythrocyte-rich layer as a percentage of the overall volume of the mixture). Histological examination in a previous study showed that the resulting RBC concentration (measured by area) is slightly different.15 For clarity, the percentage area covered by RBCs observed histologically will be referred to as %a.
In addition to these five clot types, clots were also manufactured from whole blood, which produces a RBC-rich clot (approximately 91%a), which is histologically similar to the 80% RBC clots (approximately 99%a). Clots were prepared in 40 mL vials filled with 30 mL of blood mixture, and clotting was initiated in the vial by adding a 2.06% calcium chloride (CaCl2) solution to reverse the action of the sodium citrate, and subsequently incubating at 37°C for about 30 min. These are referred to as whole blood clots in this article. Clots were stored at 4°C for <24 hours before testing.
Sample preparation
Before testing, the mass of clot material in each vial was recorded to establish a baseline mass value with nominal 100% moisture content. This was done in order to track the relative moisture loss of the samples during the experiment, as clot samples tend to lose mass through gradual loss of fluid over time. Clot samples were then removed from their vials and lightly blotted using absorbent tissue, to remove latent moisture. Test samples were prepared by cutting the clot into sections of an approximate height of 8 mm, which were then shaped into 20 mm diameter cylinders using a biopsy punch (figure 1) to produce samples with consistent volumes and surface areas. The average sample mass was 2.01±0.32 g. This resulted in four or five ‘replicate’ samples per vial, depending on the size of the source clot slug. Each sample was placed on an individual sample dish and labeled for tracking throughout the experiment. The mass of each clot was monitored throughout the experiment in order to track the moisture content in comparison with the nominal 100% baseline value.
(A) Example of a 20 mm test sample (80% red blood cell (RBC) content). (B) Example of a 0% RBC (fibrin-rich) content test sample.
Friction testing
A custom-made testing apparatus was used for friction testing of the clots, which is shown in figure 2. The fixture incorporates a low-friction test surface comprising a dry polytetrafluoroethylene (PTFE)-coated cold rolled stainless steel plate, mounted on a customized fixture that allows a controlled change in surface angle. The clot samples were placed on the surface and the angle was slowly increased. Clots were observed until they began to slide on the inclined plate, and the sliding angle was measured using a digital inclinometer (DWL-80Pro). Each experiment was recorded and the sliding initiation point was confirmed through video analysis. After each test, the surface was wiped clean using water and tissue.
Illustration of custom test setup for friction testing. (A) Polytetrafluoroethylene (PTFE)-coated stainless steel (SS) plate. (B) Test setup with plate inclined. (C) Diagram for calculating the coefficient of friction: m=mass, g=gravity, R=normal reaction force, F=friction force, θ=angle of inclination at moment of slippage.
Each replicate was tested on the inclined surface at least five times (resulting in a total of 20–28 tests per test group, depending on the number of replicates yielded for each clot slug). Testing was conducted at room temperature (21.5°C±1°C).
A standard friction calculation was carried out to characterize the coefficient of static friction of each sample, by calculating the tangent of the angle at which sliding motion started. This calculation is based on the forces depicted in figure 2C, where θ is the angle of inclination at the moment the sample begins to slide. The coefficient of friction µ is related to the sliding initiation angle by:

µ is reported as a friction value throughout the publication for simplicity, although the parameter being measured may also include an adhesive interaction between the clot and the surface in addition to friction. The specific measurement of an adhesive interaction is beyond the scope of this study, and may merit future research.
Friction testing on arterial surface
Friction testing was performed using two clot types on the inner lumen of an artery, and these data were compared with results from the PTFE surface testing. Bovine aortic tissue was obtained from a local veterinary facility (Ash Stream vets, Co. Mayo, Ireland) and transported to the laboratory, where it was stored at 4°C until testing. The aorta was split longitudinally and opened out, in order to create a flat testing surface. This surface was anchored to the inclining plate, and the test was conducted as described above (n=6 for each test group). The arterial surface was misted with saline over the course of the experiment in order to keep it hydrated. The two clot types used were a 0% RBC clot, and a clot formed from whole blood (termed whole blood clots).15
Statistical analysis
The mean, SD, and 95% CI around the mean were calculated for each test group. Statistical significance between groups was established through a one-way analysis of variance, followed by a Tukey's honestly significant difference pairwise comparison. Differences between groups were deemed statistically significant for p<0.05.
Results
Friction testing results
The results of the friction tests of clots of varying composition are summarized in table 1. Variability in the coefficient of friction was highest in the 0% RBC group (which has a relatively high SD) and decreased with increasing RBC content, with the lowest in the 80% RBC group.
Coefficient of friction of clots of varying composition
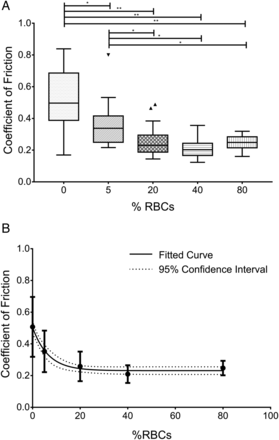
The data are shown graphically as a box plot in figure 3A. A statistically significant difference was found between the 0% group and all other groups, as well as between the 5% group and all other groups, respectively. However, no statistically significant difference was found between the coefficient of friction of the 20%, 40%, and 80% groups.
(A) Box plot representing the coefficient of friction for each clot composition. *p<0.05, **p<0.0001. (B) Coefficient of friction by clot composition with a fitted curve showing a trend towards decreasing friction coefficient with increasing red blood cell (RBC) content. Markers indicate mean, bars indicate SD, solid line shows one-phase exponential decay fit, and dotted line shows 95% CI.
This demonstrates a change in behavior for clots containing reduced levels of RBCs, which show high levels of friction, compared with clots containing high levels of RBCs, which show low levels of friction. Furthermore, clots with high RBC content show similar levels of friction to each other. Figure 3B shows the data with a fitted line to represent the trend towards decreasing friction with increasing RBC content (exponential one phase decay, R2=0.46).
A notable feature of the fibrin-rich clots (0% RBCs) is their inability to retain moisture in the same way as RBC-rich clots. We noted consistent reduction in mass from initial clot preparation to the time the clots were tested. The mass of the pure fibrin clots reduced to 41% of the baseline value when the latent moisture was removed through blotting at the start of the experiment. Clots containing RBCs reduced to 67–81% of their baseline mass after blotting. The effects of moisture loss on the coefficient of friction for the fibrin-rich clots were not consistent (figure 4), and no clear trend between moisture loss and coefficient of friction could be detected for this experiment. This may, in part, explain the large variation in coefficient of friction for the 0% RBC clots, compared with the relatively consistent results for the 80% RBC group (figure 4B).
Examples of the relationship between mass change due to moisture loss over the course of the friction testing and coefficient of friction, by clot type. Change in mass is indicated by (M%)—for example, 0% M41%=clot type 0% red blood cell (RBC) at 41% of original mass. n=5 per group. (A) 0% RBC. (B) 80% RBC. *p<0.05.
Results of friction testing on aortic surface
The coefficient of friction of two clot types (fibrin-rich and RBC-rich) tested on an aortic surface are shown in figure 5. The 0% RBC (fibrin-rich) clot has a statistically significantly higher coefficient of friction than the whole blood (RBC-rich) clot, which is in keeping with the results of the previous section. The 0% RBC clot had a coefficient of friction approximately three times higher than that of the whole blood clot, in comparison with our previous experiment on a low friction surface, where the 0% RBC clots had approximately double the value of coefficient of friction of the 80% RBC clots. The overall net values of coefficient of friction are higher for the aortic surface than for the low-friction surface used in the experiment.
The coefficient of friction of 0% red blood cell (RBC) and whole blood clots tested against an aortic surface. *p<0.001.
Discussion
We hypothesized that clots of varying composition may exhibit different levels of friction, which might contribute to their relative resistance to clot removal during thrombectomy. To demonstrate this, we created a range of clots which varied in composition from 0% RBC content to 80% RBC content. The results in figure 3 clearly show that clot composition does affect the coefficient of friction. Clots with low RBC content (and high fibrin content) tend to have a much higher coefficient of friction than clots with high RBC content. There is a statistically significant difference between the coefficient of friction of the 0% RBC group and all other groups, as well between the 5% RBC group and all other groups. Interestingly, there is no statistically significant difference between the 20%, 40%, and 80% groups. This suggests a ‘threshold’ value of RBC content (in the region of 20%) above which there is a plateau in friction properties, and below which there is a dramatic increase. Of the 50 clots analyzed by Liebeskind et al,13 12 had <20% RBC content (which is around 24% of the sample population). It is likely that the number of fibrin-rich clots present in patients with stroke is higher than this, as a portion of the clots that are not retrieved may also be fibrin rich.
The test results on a PTFE surface are supported by additional testing, which was conducted for two clot types on an arterial surface. The same trend can be seen as on the low-friction testing surface, where fibrin-rich (0% RBC) clots have a much higher coefficient of friction (fibrin-rich clots had a coefficient of friction approximately three times higher than RBC-rich clots on the arterial surface, and around double on the PTFE surface). The absolute values of the coefficient of friction measured against the artery are higher than those measured against PTFE. However, this is expected as PTFE is designed to be a low-friction surface. Furthermore, although the bovine aorta used was fresh, and regularly sprayed with water to maintain moisture, it is possible that manipulation of the surface might have damaged the endothelial layer, resulting in sliding contact between the clot and the exposed connective tissue layer beneath. The presence of small side branches might have also increased the irregularity of the arterial surface; however, care was taken to avoid regions with irregularities such as these during testing.
Research into the correlation between imaging and clot composition has been performed by several groups, with a view to predicting clot composition before thrombectomy.11 ,13 ,19 The information presented here may be relevant in predicting the clot properties through imaging techniques, as characterizing individual clots is time-consuming and technically challenging, whereas determining whether a clot falls above or below a certain threshold may be more clinically feasible.
Liebeskind et al13 demonstrated in their study that the hyperdense middle cerebral artery sign (HMCAS) in CT was present in cases where clots contained an average of 47% RBCs, and blooming artifact in MRI was present in those with an average of 42% RBC content. Interestingly, in relation to the results of this study, the average RBC contents where HMCAS and blooming artifact were absent were 22% and 23%, respectively. In a recent review and meta-analysis by Brinjikji et al,20 the authors show that this result is confirmed by five other publications. We have shown that at RBC content <20%, the friction of the blood clots starts to increase significantly. This information could potentially be used in conjunction with our report to detect high friction clots, and thus to aid in procedural decision making. Brinjikji et al21 have shown that in five out of seven publications correlating hyperdense artery sign (HAS) and recanalization, the presence of HAS (which is an indicator of high RBC content) is a predictor of better recanalization rates. The relationship between clot composition, imaging, and recanalization rates clearly merits further research.
A further observation of this study is the reduced ability of fibrin-rich clots to retain moisture compared with RBC-rich clots. This may in part explain the higher levels of variability seen in the coefficient of friction in the 0% and 5% RBC groups. Although there is some suggestion in our results that moisture loss is an influence on the coefficient of friction for the fibrin-rich groups, there is no clearly defined trend. The reason for this rapid moisture loss in the fibrin-rich groups may be related to the microstructure of the clots. Fibrin-rich clots are made up of a network of fibrin fibers interspersed with platelets. RBC-rich clots have a similar fibrin matrix, but with RBCs trapped within the mesh. This structure may have a higher capacity to retain moisture than the fibrin-dense structure. Mehta and Nogueira22 identified the differences in microstructure between early and mature thrombus through scanning electron microscopy. They showed that mature and organized thrombi had a highly dense and tightly integrated structure, while young thrombi had a much looser structure. There is some reason to believe that the moisture loss of the fibrin-rich clots might be representative of the aging of the clot, as we have seen in bench testing that as the clot becomes more mature, it becomes denser and loses fluid. Whether this effect of densification bears any relation to mechanical compression of the clot (eg, through a failed pass with a stentriever) remains to be determined.
Limitations
One limitation of this study is the homogeneous nature of the clots, as they are prepared in a controlled environment in the laboratory. This may make the clots slightly less clinically representative, as clots retrieved from patients tend to have irregular structures. However, for the purposes of a mechanical test, it is useful to use a homogeneous sample set which allows for repeatable and comparable tests based on the clot composition. Furthermore, the composition was measured in terms of RBC and fibrin content, without measuring platelet or white blood cell content. However, controlled protocols and systematic preparation of the clot should result in similar platelet and white blood cell content in each clot analog type.
It was also observed in this test that the clots tended to lose moisture over the course of this experiment. However, we attempted to compensate for this by cutting the samples to a consistent size and shape directly before the test, and tracking the mass of the clot over the course of the experiment. In this way, the clot samples all had similar surface contact areas and masses at the time of testing, despite relative moisture loss.
The clots in this experiment were tested on a synthetic surface. As a verification of our methods, we tested fibrin-rich and RBC-rich clots on a bovine arterial surface, and found a similar response. However, the decision was made to use a synthetic surface for the test in order to mitigate variability and provide a true comparative test between clot compositions. Furthermore, it is possible that in the case of clots obstructing an artery, there is artery–clot adherence which also contributes to the ‘friction’ of the clot, as exemplified by the high friction interaction measured on the bovine aorta surface. This interaction merits further investigation.
Finally, in this study we investigated only one property of clot, while in all likelihood there are several factors (such as compression) in play which contribute to the difficulty of clot retrieval. Further investigation into the properties of clot is merited in the future.
Conclusion
The ratio of RBCs to fibrin in clot composition does affect the frictional properties of clot. We have shown that clots with <20% RBC content have a much higher coefficient of friction. This could potentially be useful information in the design of devices for patients with acute ischemic stroke.
References
Footnotes
GMG and KM contributed equally.
Contributors GMG: data analysis and interpretation, as well as manuscript writing. KM: experimental conception and design, the preparation of clots, data collection, analysis, and interpretation. MM: testing and data collection. SD: clot preparation, data collection, and data analysis. MG: experimental conception and design, data interpretation, and manuscript drafting and editing. PAB: experimental conception, testing, data interpretation, and manuscript drafting and editing. All authors provided suggestions and feedback and approved the final manuscript.
Funding This work was supported by Neuravi Ltd.
Competing interests GMG, KM, MM, SD, and MG: other work from Neuravi Ltd, outside the submitted work. PAB: personal fees from Neuravi, Stryker, Codman DePuy Synthes and Medtronic, outside the submitted work.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Please contact the corresponding author with data sharing requests.