Article Text
Abstract
Introduction Optical coherence tomography (OCT) provides high resolution imaging of tissue; this technology has been validated using intra-arterial catheters in the evaluation of arterial anatomy, pathology and treatments. The perforating cerebral arteries and intracranial stents have not been previously visualized with an OCT catheter.
Methods Using a standard transfemoral endovascular technique, a LightLab C7 Dragonfly catheter was inserted in the middle and posterior cerebral arteries of a fresh frozen cadaver. OCT images of the cerebral vessels and a deployed Pipeline Embolization Device were acquired using the LightLab C7-XR OCT Intravascular Imaging System.
Results Distal cerebral artery access with the imaging catheter was feasible via the femoral artery using a distal access catheter instead of the standard monorail system used in coronary investigations. Imaging of perforators and stent struts had exceptional resolution.
Conclusion The first use of a commercial OCT catheter in the evaluation of intracranial vessels using transfemoral endovascular techniques is described. Challenges of intracranial OCT include blood clearance and vessel tortuosity. This technology may aid in the diagnosis and treatment of cerebrovascular disease in the future.
Statistics from Altmetric.com
Introduction
Neuroendovascular surgery is an area of rapid growth in the diagnosis and treatment of cerebrovascular disease but visualization of small intracranial branches and perforators is limited. Intravascular imaging has become routine in coronary intervention, and endovascular optical coherence tomography (OCT) has successfully detailed coronary artery anatomy, pathology and treatments, including stents.1–3 Intravascular OCT allows tissue evaluation with better than 10 μm resolution,4 and the accuracy has been validated with histology in other studies.5 6
Perforating arteries within the subarachnoid space are end arteries to the perforated substance, and occlusion can cause infarction. The outer diameter of these vessels averages 300–400 μm and can be as small as 70 μm in outer diameter.7–9 The high resolution of OCT may evaluate these small but critical arteries better than currently available imaging techniques, including intravascular ultrasound and digital subtraction angiography.1 Prior to the current study, OCT imaging of the intracranial vessels has been limited to the vertebral and carotid arteries where there are few vessels not visible using routine angiography.
This study describes our initial experience using neuroendovascular access for intracranial navigation of an OCT catheter to image the vessels of the circle of Willis in a human cadaver. We used a standard endovascular technique and commercially available catheters to navigate an OCT catheter beyond the carotid siphon and vertebrobasilar junction. In this feasibility study, attention was given to perforator imaging and imaging within a deployed intracranial stent.
Materials and methods
In the first part of the study, the femoral artery was catheterized in a fresh frozen human cadaver. Using a 6 French Envoy guide catheter (Cordis Neurovascular, Miami Lakes, Florida, USA), the left internal carotid artery was accessed. A rotating hemostatic valve connected the guide catheter to a continuous saline flush at approximately 100 mm Hg. The C7 Dragonfly Intravascular Imaging Catheter (0.036 inch outer diameter, LightLab Imaging Inc, St Jude Medical, St Paul, Minnesota, USA) was advanced along a microwire monorail system according to the commercial device's design. The imaging catheter could not be advanced beyond the carotid siphon. The catheter was also easily damaged during navigation and subsequently unable to generate adequate images.
A distal access catheter (0.044 inch inner diameter, 115 cm length; DAC, Concentric Medical, Mountain View, California, USA) was advanced into the middle cerebral artery (MCA) using standard endovascular technique. The imaging catheter could be advanced through the DAC without using the monorail wire. The imaging catheter was subsequently unsheathed inside the MCA. OCT images were obtained in accessible segments using the C7-XR OCT Intravascular Imaging System (LightLab). To obtain clear images, residual cadaveric blood products were flushed with contrast injection through the guide catheter during each image acquisition sequence. The rotating and translating light source and imager moved through the imaging catheter between 2.5 and 5 mm/s, creating 100 frames/s over 15–30 mm. After deployment of the Pipeline Embolization Device (Covidien, Mansfield, Massachusetts, USA) in the basilar artery, the same technique was used to image the posterior cerebral arteries and the stented basilar artery.
In the second part of the study, the brain and intracranial vessels were removed from the cadaver en bloc. The remaining arterial tree was evaluated with OCT through direct introduction of the imaging catheter and saline flush into the sectioned termini of the carotid and vertebral arteries.
Results
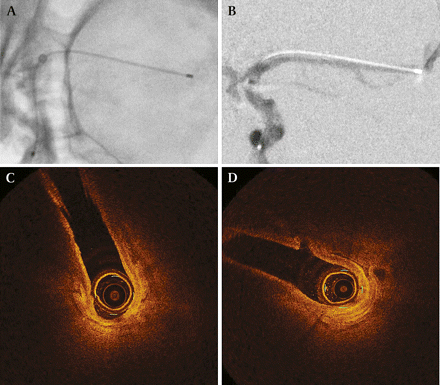
The Dragonfly imaging catheter was advanced to the MCA bifurcation and into the posterior cerebral arteries using the modified access technique with a DAC. OCT revealed the anatomy of the arterial origins seen on angiography and revealed perforating vessels, which could not be seen with angiography (figure 1). Pressurized saline irrigation provided satisfactory flushing for imaging but inadequate cadaveric vessel distention, and non-uniform rotational distortion due to cerebral vessel tortuosity altered OCT quality in some frames. One imaging catheter broke during imaging through the internal carotid artery terminus. Despite these challenges, image quality was excellent overall.
Transfemoral endovascular optical coherence tomography. (A) Fluoroscopic imaging shows the tip of the imaging catheter near the middle cerebral artery (MCA) bifurcation. (B) Digital subtraction angiography demonstrates the MCA with the imaging catheter in position. Optical coherence tomography imaging shows (C) the takeoff of the early temporal branch seen in (B), and (D) a perforating vessel, 0.21 mm in diameter, which could not be seen on angiography. The MCA is not fully distended due to inadequate pressure in the cadaveric vessels.
Following Pipeline Embolization Device deployment in the basilar artery, OCT revealed the relationship of all 48 strands of the stent to the vessel wall. The stent had excellent strut apposition. Major branches and perforators were visualized at their takeoff from the basilar artery, and stent struts across these orifices could be analyzed (see video, available online only). The C7-XR system measured the diameter of the vessel around the stent (figure 2).
Optical coherence tomography imaging of a pipeline embolization device deployed in the basilar artery. (A) Apposition of the stent struts is evaluated, and a perforating artery can be seen exiting from the vessel wall (bottom). (B) Another perforator (bottom) can be seen exiting toward the brainstem (left). Non-uniform rotational distortion causes blurring when the light catheter bends (bottom right). (C) LightLab software evaluates the average diameter (3.44 mm) and area (9.24 mm2) of the artery after stenting with a 3.75 mm diameter stent.
Once feasibility of the transfemoral endovascular OCT had been established, ex vivo imaging was performed in the anterior, middle and posterior cerebral arteries bilaterally (figure 3). The second part of the study provided outstanding imaging of the perforating vessels, albeit under optimal and non-physiological conditions. Perforators were commonly seen exiting the parent artery at oblique angles; they often reversed direction near their origins. (For MCA perforator, anterior communicating artery complex and pipeline embolization device OCT imaging video, refer to the online supplement.)
(A) Perforating artery exits the middle cerebral artery (MCA) (left) and is seen in cross section passing back through the frame. The average outer and inner diameters are 0.49 mm and 0.27 mm, respectively. (B) Another perforator exits the MCA obliquely (right) with an inner diameter of 0.12 mm. Artifact from incomplete saline flush and an air bubble (top) are seen within the vessel lumen. (C) Optical coherence tomography shows a cross section of a 1.0 mm diameter posterior communicating artery. Optical coherence tomography demonstrates at least one perforator exiting the vessel.
Discussion
To our knowledge, the present study offers the first minimally invasive evaluation of the perforating branches of the cerebral arteries and cerebral stents. Additionally, this is the first use of a commercially available OCT system to assess the intracranial arteries. The coaxial DAC and C7 Dragonfly Intravascular Imaging catheter combination was a successful alternative to the standard wire monorail technique for inserting the imaging catheter into distal cerebral vessels in this particular case. Regarding the highly variable anatomy of the intracranial vessels, however, we do not assume this technique would be universally effective in gaining imaging catheter access.
The images obtained with intraluminal OCT provide real time ‘histological’ evaluation unparalleled by any other imaging modality. Other investigators have demonstrated the capacity of intracranial OCT imaging, but to our knowledge, imaging of the basilar and cerebral arteries using OCT technology has never been published. Thorell et al applied OCT technology to canine extracranial carotid arteries by examining coiled aneurysms post mortem.10 Mathews et al evaluated the cervical, petrous and cavernous segments of human cadaveric carotid arteries with OCT using standard transfemoral endovascular techniques, validating findings with histology.5 11 Their group evaluated the carotid and vertebral arteries in living patients, including one with a treated aneurysm.12 After eliminating intraluminal blood products, distortion and access challenges, the second part of our study demonstrated the potential of intracranial OCT by providing excellent anatomical detail.
We have shown that the resolution of OCT is adequate for visualizing the smallest perforating branches and the strut apposition of intracranial stents. Furthermore, we have demonstrated an alternative coaxial technique using the C7 Dragonfly Intravascular Imaging Catheter, which could eventually be translated to living subjects using standard techniques and commercial devices.
There are many limitations to this technology in its current state, preventing routine use in living subjects. Firstly, blood clearance is inadequately addressed in this feasibility study. Endovascular OCT requires light transmission to and from the vessel wall, but intraluminal blood products absorb light, prohibiting transmission. Static cadaveric blood products under low pressure were easily flushed with a contrast bolus allowing imaging over the entire 6 s imaging interval. In living subjects, the blood products may be thicker, and the pressure and flow dynamics of blood will likely make adequate clearance more difficult. While blood clearance in coronary artery studies has been successful, intracranial imaging may be more challenging due to collateral circulation through the circle of Willis. Furthermore, the additional contrast load delivered for every imaging sequence may add a risk that is not overcome by the benefit of OCT. An equally viscous non-toxic substance could resolve this concern.
A second limitation we encountered was imaging within tortuous anatomy. The images shown from the first part of the study are from relatively straight vessels, the middle cerebral and basilar arteries. Ideal analysis requires the imaging catheter be translated in a straight line providing sequential, parallel slices through the vessel. However, intracranial pathology is certainly not limited to straight vessels. We encountered non-uniform rotational distortion within the basilar artery (figure 2B), and we expect this distortion to be much worse in segments such as the carotid siphon. Furthermore, an imaging catheter broke and was discarded after imaging was attempted through the internal carotid artery terminus.
Despite current limitations, endovascular OCT provides a promising tool for evaluation of cerebrovascular disease, including stenosis and aneurysms. Knowledge of perforator density and diameters could help neurointerventionalists determine the optimal location for stent deployment, and further studies could elaborate ideal stent strut density for stents deployed over perforators. Intracranial stent apposition and endothelialization may be important predictors of vessel healing,3 13 and OCT has the potential to evaluate vessels after stenting to allow more tailored post-stenting therapy.
Conclusion
Acquisition of OCT images of cerebral vessels using catheters through a transfemoral approach is challenging but feasible. We have described a neuroendovascular approach using a distal access catheter in coaxial fashion that permits navigation of a commercial OCT catheter for evaluation of perforating arteries and stent struts. Images of these tiny perforators and stents were superior to available non-invasive and minimally invasive modalities. This technology has tremendous potential in diagnostic applications for cerebrovascular disease, but limitations exposed in this preclinical trial must be addressed before translation to the clinical setting.
Acknowledgments
The authors thank St Jude Medical for lending the LightLab imaging catheters and analysis system for use in the study, and specifically Chris Brushett's assistance in troubleshooting the OCT system and creating the pictures and videos presented herein. The authors thank Joel Delacruz and the neurointerventional team for facilitating the project, and Chris Jones and the Rush University anatomy lab for assistance with cadaver care.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Download Supplementary Data (PDF) - Manuscript file of format pdf
Footnotes
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.