Article Text
Abstract
Objective The authors aimed to determine the ability of resting-state functional connectivity MRI (fcMRI) to lateralise/localise the epileptogenic zone in patients presenting with mesial temporal lobe epilepsy (MTLE) at the individual level.
Methods Basal functional connectivity (BFC) was evaluated in each hemisphere of 22 MTLE patients. 200 volumes were acquired using a single-shot GE-EPI sequence during a resting period of 10 min at 1.5 T. The signal time-course was extracted from 10 regions of interest (ROIs), five ROIs in each hemisphere, usually involved in epileptogenic networks of MTLE. Normalised correlation coefficients between pairs of ROIs signal time-courses were computed to reflect BFC. Based on normative BFC values obtained from 36 controls, the number of BFC decreases and increases were determined in each hemisphere for each patient.
Results BFC decreases were found bilaterally, although the number of decreased links was significantly higher in the epileptogenic side (p=0.025). Conversely, BFC increases were found almost exclusively in the contralateral lobe leading to a strong test effect for locating the non-epileptic lobe with a sensitivity of 64% and a specificity of 91% (p<0.001). The most frequently disconnected areas were the entorhinal cortex and the anterior hippocampus in the epileptic lobe, while contralateral BFC increases involved preferentially hippocampus and amygdala.
Conclusions This study demonstrates that the presence of BFC increases in the non-epileptic side was paradoxically the most specific marker of epileptogenic zone localisation, and suggests that a single resting-state fcMRI could be useful in the presurgical assessment of MTLE at an individual level.
- Functional connectivity
- temporal lobe epilepsy
- resting state fMRI
- clinical neurology
- epilepsy, surgery
- functional imaging
- MRI
Statistics from Altmetric.com
- Functional connectivity
- temporal lobe epilepsy
- resting state fMRI
- clinical neurology
- epilepsy, surgery
- functional imaging
- MRI
Introduction
Since the early 1990s, fMRI has been widely used as a mapping tool of brain function by measuring task-related BOLD variations. During the last decade, the neuroimaging field has witnessed a dramatic evolution of fMRI after the discovery of spontaneous fluctuations of BOLD signal at rest exhibiting coherent variations in regions belonging to the same functional networks.1 2 Such coherent slow-frequency fluctuations are at the basis of functional connectivity MRI (fcMRI) measurements and have helped to bring new insights into the complex brain network organisation.3 4 Moreover, the clinical impact of neurological disorders may be related to disorganisation of such complex networks, justifying the need for non-invasive characterisation of functional connectivity.
In mesial temporal lobe epilepsy (MTLE), using fcMRI it has already been demonstrated that cognitive impairment associated with temporal lobe epilepsy (TLE) may be linked to specific cognitive network disturbances. In particular, Waites et al showed that language impairment was related to a functional connectivity decrease between regions belonging to the language network previously defined by task-related fMRI.5 In addition, using fcMRI during a memory task, Addis et al demonstrated functional disconnections of autobiographical memory network in MTLE patients with hippocampal sclerosis (HS).6 Such studies are in favour of a relationship between altered functional connectivity and cognitive dysfunctions associated with MTLE. However, although such functional networks are closely related/connected to the epileptogenic zone (EZ, ie, responsible for seizure onset), they do not correspond to the EZ itself that mainly involves mesial temporal lobe structures.
Nonetheless, in drug-resistant partial epilepsies, the main goal of epilepsy surgery is to locate and remove the EZ. That is why, in a recent study using resting-state fcMRI, we studied the ability of fcMRI to define functional connectivity alterations in the EZ.7 In this previous study, a homogeneous group of left MTLE patients was investigated, and basal functional connectivity (BFC) decreases were found within the EZ, associated with a concomitant BFC increase in contralateral lobe at the group level. However, the stringent selection criteria and the restricted number of patients did not answer the question of the clinical relevance of BFC alterations from an individual point of view which was addressed in the present work. Here, the main goal was to define the individual usefulness of resting state fcMRI in the presurgical evaluation of drug-resistant TLE. To this aim, we studied a larger and less selected (in term of handedness and epilepsy laterality) cohort of patients (n=22) compared with controls (n=36), and determined the sensitivity and specificity of altered connectivity as a test to lateralise/localise the epileptogenic network at the individual level. We also sought to determine individually the extent, types and significance of such functional connectivity alterations.
Subjects/materials and methods
Subjects
Fifty-eight subjects (22 patients and 36 controls) gave their informed consent to be included in this study approved by the local Ethics Committee of Marseille Public Hospital.
The 22 patients (10 with right MTLE, five females; and 12 with left MTLE, seven females) were selected after a comprehensive non-invasive presurgical evaluation. Though exhibiting different types of pathology on conventional MRI, all other data (ie, interictal and ictal video-EEG (especially ictal electro-clinical semiology), interictal FDG-PET, interictal±ictal SPECT were compatible with a seizure onset in the mesial temporal lobe structures. Thus, as defined in a previous work using SEEG,8 we selected these patients, as they presented homogeneous electro-clinical features compatible with MTLE.
Clinical characteristics of MTLE patients are reported in table 1.
Clinical characteristics of mesial temporal lobe epilepsy patients
The 36 healthy controls (16 females) matched for sex (p=0.455) were free of neurological disease.
In the two groups, handedness was controlled (for patients: left-handed n=6, age=40 ±11 and right-handed n=16, age=38±13; with no age differences between the two groups: Kruskal–Walis p=0.740; for controls left-handed n=10, age=30±11 and right-handed n=26, age=29±9; with no differences between the two groups: Kruskal-Walis p=0.791).
Neuropsychological testing
Normative evaluation of memory capacity and intelligence scale of patients were assessed by the Wechsler Memory Scale—Third Edition (WMS III) (reported scores in this paper are: immediate memory quotient, IMQ; delayed memory quotient, DMQ; and working memory quotient, WMQ) and the WAIS III (Wechsler Adult Intelligent Scale—Third Edition) (reported scores in this paper are: full scale IQ, verbal IQ and performance IQ). For both tests, the normal mean values were at 100 and abnormality cut-off at 85 (−1.5 SD).
Conventional MRI
All subjects underwent a MRI examination (duration: 80 min) in the framework of a multimodal MRI protocol on a 1.5 T Magnetom Vision plus MR-scanner (Siemens, Erlangen, Germany).
Conventional MRI included T1-weighted images (TE/TR=15 ms/700 ms, 23 contiguous slices, 5 mm slice thickness, field of view (FOV) 240 mm, matrix 256) acquired in the AC-PC plane, T2-weighted images (TE/TR=112/7308 ms, FOV 240 mm, matrix 256, 23 contiguous slices, 5 mm slice thickness) acquired in the bihippocampal plane, T1-weighted inversion recovery images (TE/TR=60/8000 ms, TI=350 ms, FOV=240 mm, matrix 512, 5 mm slice thickness), FLAIR images (TE/TR=110/8000 ms, TI=2500 ms, FOV=240 mm, matrix 256, 5 mm slice thickness) acquired in a coronal axis perpendicular to the bihippocampal plane, and sagittal 3D-MPRAGE images (TE/TR=4/9.7 ms, isotropic voxel of 1.25×1.25×1.25 mm3).
Resting state fcMRI
Data acquisition and processing
Two hundred brain volumes (time elapsed between blocks: 4 s) were acquired using a single-shot multislice gradient-echo echo-planar imaging (GE-EPI) sequence (TE 55 ms, TR 4 s, 30 contiguous slices, 4 mm thickness, matrix 64, FOV 256 mm). Subjects were instructed to simply keep their eyes closed and to not fall asleep.
Data processing
Resting-state fcMRI acquisitions were preprocessed using SPM2 software (Welcome Institute, London). After slice timing correction, images were realigned before spatial normalisation (16 non-linear registration 7×6×7 basis functions) and smoothing (12 mm). Sources of spurious or regionally non-specific variance related to physiological artefacts were removed using a method previously described.7 10 11 We used a regression including the CSF signal (averaged over the lateral ventricles) and the white matter signal (averaged over a region centred in the deep cerebral WM) in order to reduce the non-neuronal contributions to BOLD correlations.7 10 11
Regions of interest
According to the methodology described in a previous study,7 10 ROIs (five in each hemisphere) usually involved in epileptogenic networks of mesial TLE were defined. These regions were automatically obtained from a digital Talairach atlas (Pick atlas toolbox, SPM2) and consisted of amygdala (Amy), entorhinal cortex (EC), anterior hippocampus (AntHip), posterior hippocampus (PostHip) and temporal pole (Brodmann area 38, BA38).7 These ROIs were used as masks applied onto the residual images to extract the mean signal time-courses from each predefined ROI.
To determine functional interactions between ROIs in each temporal lobe, correlation coefficients between pairs of signal time-courses were computed (JMP statistical software). Correlation coefficients were then normalised using the Fisher transformation, rN =0.5log[(1+r)/(1−r)], to reflect BFC and to perform subsequent statistical analyses.
Normative BFC values in controls
For each control group (left and right-handed), unilateral BFC values were tested against the null hypothesis for each link (Mann–Whitney test, corrected p<0.005 (10 comparisons per group)) to determine the statistical significance of each correlation. BFC values for non-significant correlations were set to zero. The within-group effect of BFC was tested for the left- and right-handed control group for each link (Wilcoxon signed rank).
For each subgroup, differences in BFC values between contralateral links were also tested (Wilcoxon rank test, corrected p<0.005 (10 comparisons per group)).
For each hemisphere, between-group (left-handed and right-handed controls) comparisons of BFC values were performed for each link using a Mann–Whitney test. A corrected p<0.005 was considered to account for the 10 comparisons.
BFC at individual level in patients
In order to evaluate the added value of BFC at the individual level, z scores were calculated for patients relative to the mean and SD of the corresponding subgroup of controls. To limit the potential bias related to possible variations in structural and functional asymmetries of temporal lobes, we calculated z-scores for patients relative to control group with the same handedness. Each connection from each hemisphere was classified as abnormal for z score values above or below 2 for each subject, and the numbers of BFC decreases and increases were obtained for each hemisphere of each patient. In controls, this cut-off of 2 SD included the 90th percentile for each link studied and for the two groups of controls (right- and left-handed).
Sensitivity and specificity of BFC alterations to locate the epileptogenic hemisphere
Two types of connectivity alterations were evaluated: (1) the presence of at least one link showing significant BFC decrease relative to controls in the epileptogenic temporal lobe; (2) the presence of at least one link with significant BFC increase relative to controls in the contralateral temporal lobe.
For these two criteria, we classified BFC decreases and increases from each hemisphere of each patient as true positive, true negative, false positive and false negative. Then, we assessed: sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and the power of these tests using the Yule Q coefficient, χ2 and Yuden index. The two criteria tested to classify the epilepsy lateralisation were intentionally very simple and were defined a priori. No attempt to optimise these criteria was conducted on the present dataset to limit artificial overfitting.
Relationships between BFC and clinical data
In patients, pairwise correlations were performed in order to determine a potential relationship between BFC and: (1) neuropsychological scores; (2) seizure frequency; and (3) disease duration. We considered that each test aimed at answering a single different question; consequently, no multiple comparison corrections were applied (Spearman rho, p<0.05).
Results
Neuropsychological data in patients
Neuropsychological data are reported in table 2. Twenty-one out of the 22 patients (10 with right MTLE and 11 with left MTLE) performed the whole preoperative neuropsychological evaluation.
Neuropsychological characteristics of mesial temporal lobe epilepsy patients
In MTLE patients, scores for WAIS III were significantly lower than normative values for the three reported quotients: full-scale IQ (p<0.001), verbal IQ (p<0.001) and performance IQ (p<0.001).
Assessment of WMSIII showed that immediate memory (IMQ) and delayed memory quotients (DMQ) were not significantly altered (respectively p=0.861 and p=0.805), and the same result was observed for the two subtests (auditory IMQ p=0.206; visual IMQ p=0.352; auditory DMQ p=0.494; visual DMQ p=0.424). In contrast, the working memory quotient was significantly below normative values (p<0.001), as well as the auditory (p=0.035) and visual (p<0.0001) working memory subtests. Variables such as EZ laterality and handedness did not interfere with all quotients (WAIS III and WMS III, Wilcoxon Sign-Rank test). In addition, we did not find any significant discrepancies between visual and auditory subtests in patients (p=0.765 in right MTLE, and p=0.680 in left MTLE using a paired test (Wilcoxon test)).
Normative BFC in controls
In right-handed controls, significant BFC was present bilaterally for all the possible links (p<0.05) except for the link EC-PostHip in the two hemispheres. Left–right asymmetry in BFC was present, with a left predominance for the links AntHip-PostHip, Amy-AntHip and Amy-PostHip, while a right predominance was observed for the link Amy-EC (p<0.005).
The same significant links were observed in left-handed and right-handed controls. Left–right asymmetry in BFC was present with a left predominance for the link Amy-AntHip, while a right predominance was observed for the link Amy-EC (p<0.005).
Relative to the left-handed group, the right-handed controls showed a BFC increase in the left temporal lobe for the links Amy-PostHip and AntHip-PostHip (p<0.005) (figure 1). Group data are reported in table 3.
Differences of basal functional connectivity in controls within mesial temporal lobes depending on handedness. All connecting lines correspond to significant basal functional connectivity. Grey lines correspond to links that are not significantly different between right- and left-handed controls. Bold lines correspond to links significantly different between right and left-handed controls. Right-handed controls showed a significantly higher basal functional connectivity in the left temporal lobe for the links amygdala-posterior hippocampus and anterior–posterior hippocampi (Wilcoxon rank test, corrected p<0.005). Note that for the two groups, the link between entorhinal cortex and posterior hippocampus was not significant bilaterally.
Normalised correlation coefficients (and SD) between ROIs in each lobe, for right-handed and left-handed controls
Accordingly, individual results in patients were compared with the subgroup of controls with the same handedness.
Altered BFC in patients
Individual data are reported in table 4.
Bilateral regional occurrence of abnormal pairwise basal functional connectivity in mesial temporal lobe epilepsy patients
Two out of the 22 MTLE patients showed a normal BFC profile (patients 5 and 11). BFC decreases were observed in 18 out of the 22 patients (patients 1–4, 6–8, 10, 12–18, 20–22). Sixteen had at least one link BFC decrease in the EZ (patients 1–4, 6, 10, 12–18, 20–22), while 10 had a contralateral BFC decrease (patients 1–3, 7, 8, 10, 15, 20–22). Eight had bilateral BFC decreases (patients 1–3, 10, 15, 20–22), eight had unilateral BFC decrease(s) in the EZ (patients 4, 6, 12, 13, 14, 16-18), and only two had unilateral BFC decrease(s) in the contralateral hemisphere (patients 7, 8).
Fourteen out of the 22 patients had BFC increases (patients 1–4, 9, 12–17, 19, 20, 22). Twelve had unilateral BFC increase(s) restricted to the contralateral temporal lobe (patients 1–4, 9, 13–17, 19, 20), while only two had a BFC increase in the two temporal lobes (patients 12, 22). Note that in these two patients, the number of links with BFC increase was the same or higher in the lobe contralateral to seizure.
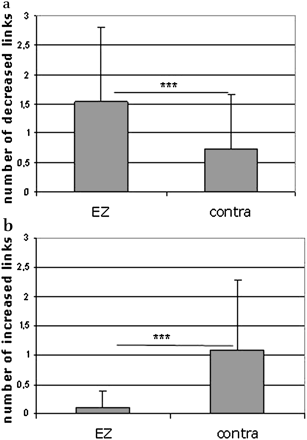
At the group level, the number of links with a BFC decrease was higher in the epileptogenic side (mean=1.545, SD=1.262) compared with the contralateral hemisphere (mean=0.727, SD=0.935) (p=0.025) (figure 2A). Moreover, the number of links with BFC increase was higher in the non-epileptogenic temporal lobe (mean=1.091, SD=1.192) in comparison with the epileptogenic side (mean=0.091, SD=0.294, p<0.001) (figure 2B).
Group analysis of altered basal functional connectivity prevalence in epileptogenic zone (EZ) lobe and contralateral lobe (contra). In (a), the number of decreased links is significantly higher in the EZ compared with the contralateral hemisphere (Wilkoxon signed rank p=0.025). In (b), the number of increased links is significantly higher in the contralateral temporal lobe to EZ compared with the EZ temporal lobe (Wilkoxon signed rank p<0.001).
Qualitative study of the most altered areas
The most disconnected structure of the epiletogenic network studied was the EC. Indeed, in the whole group of patients, we observed 25 links with significant BFC decreases involving EC in the EZ (36.76% of links with BFC decreases). The second most altered area was the AntHipp (16; 23.53%).
In the contralateral temporal lobe, BFC increases most often involved PostHipp (16; 33.60%), AntHipp (11; 22.91%) and Amy (10; 20.83%).
Diagnostic value of BFC to lateralise epilepsy
Table 5 shows the sensitivity, specificity, PPV and NPV for each test.
Sensitivity and specificity of basal functional connectivity (BFC) alterations to lateralise the epileptogenic zone (EZ)
BFC decreases were found bilaterally exhibiting a lateralisation power with a sensitivity of ∼73% and a specificity of 54.5%. Conversely, BFC increases were found almost exclusively in the contralateral lobe leading to a strong test effect for locating the non-epileptic lobe with a sensitivity of 64% and a specificity of 91% (p<0.001).
Relationship between BFC and visible lesions on conventional MRI
Thirteen patients had unilateral HS; four had cortical dysplasia, and two had other types of lesions (one temporal post-traumatic lesion and one hippocampal dysplasia). We tested (Wilcoxon rank test) the influence of MRI visible lesion on neuropsychological data and the number of altered links, by comparing data from the subgroup of 13 patients with HS and the subgroup of nine patients with no HS. No difference was observed for neuropsychological data between the HS and no HS subgroup. In addition, no significant difference was found between the two groups concerning the number of altered BFC, either the number of BFC decreases in the EZ lobe or the number of BFC increases in the contralateral lobe.
Relationships between BFC and clinical data
No significant statistical relationship was observed between seizure frequency, disease duration and BFC. However, correlating BFC values with neuropsychological memory scores in patients, we observed a significant positive correlation between working memory (WM) scores and increased BFC in the non-epileptogenic temporal lobe. Indeed, the Amy-BA38 link, contralateral to EZ, was correlated with working memory quotient (WMQ) (Spearman rank test, p=0.0494) and auditory WMQ (p=0.0413). In addition, the EC-AntHipp link contralateral to EZ was positively correlated with WMQ (p=0.0109), auditory WMQ (p=0.0043) and visual WMQ (p=0.0024). We also tested the spread between verbal and visual performance scores as a potential parameter. However, we did not found any significant discrepancies between visual and auditory subtests in patients (p=0.765 in right-sided MTLE, and p=0.680 in left-sided MTLE using a paired test (Wilcoxon test)).
Discussion
Using resting state fcMRI, we studied BFC in patients presenting with MTLE in order to test the accuracy of the method in the EZ location.
In this work, the main results were: (1) presence of a BFC alteration pattern characterised by a bilateral decrease predominant in the epileptic side, and a unilateral increase almost exclusively observed in the contralateral ‘non-epileptic’ lobe, (2) evidence that the most frequently disconnected areas were the entorhinal cortex and the anterior hippocampus in the epileptic lobe, while contralateral BFC increases involved preferentially posterior hippocampus, anterior hippocampus and amygdala, (3) at the individual level, the presence of BFC increases in the non-epileptic (contralateral) side was paradoxically the most specific marker of EZ localisation.
Basal functional MTL connectivity lateralisation depending on handedness
In right-handed controls, an asymmetrical leftward organisation of BFC was present between MTL structures. These results were discussed in a previous study7 in line with structural data obtained by quantitative diffusion tensor tractography.12 Compared with right-handed controls, left-handed control BFC was less leftward lateralised, except for the amygdala–anterior hippocampus link. This interesting finding clearly showed an effect of handedness on BFC in controls. The effect of handedness on temporal-lobe volumes has already been reported in studies using morphometry.13 However, to our knowledge, no study has investigated the handedness effect on BFC, and resting-state connectivity studies include preferentially right-handed subjects probably in order to control the handedness variable.14 Here, we demonstrate that asymmetries in brain lobes due to handedness are not only anatomical but also functional. According to this impact of handedness on BFC in controls, we controlled this variable by comparing individual results in patients to the subgroup of controls with the same handedness. However, the fact that patients might have handedness changes due to pathological plasticity must also be acknowledged.
Basal functional connectivity decreases in MTLE are not specific of EZ
In patients, at an individual level, BFC decreases were often bilateral, even if the epileptic side was preferentially affected. Therefore, not surprisingly, we found a low specificity and sensitivity of BFC decreases as a diagnostic test for EZ localisation. This is concordant with other imaging studies concerning structural as well as metabolic data. Bilateral structural abnormalities have already been shown using morphometry and diffusion-weighted imaging even in patients presenting with visible unilateral HS.15–20 In addition, bilateral metabolic abnormalities were also measured by magnetic resonance spectroscopy and positron emission tomography with fluoro-desoxyglucose in mesial temporal structures.21 22 Such abnormalities may be observed in the presence or absence of HS and may involve areas devoid of any visible lesions.16 The concordance between BFC and these metabolic abnormalities which have been demonstrated to be linked to interictal epileptiform discharges (IED) suggests a potential relationship between BFC and spikes.21 23
The most involved areas in these alterations were the EC and AntHip. These two structures have a crucial role in the epileptogenic network of TLE.16 24–26 This has been demonstrated by their participation to the dynamics of seizure onset using depth recording, and also by the atrophy that preferentially affects these structures in MTLE patients. EC and hippocampus interactions have been well known for decades, EC being the privileged relay of the afferent pathways to the hippocampus from neocortical areas.27–29 Finally, a quantified study of epileptogenicity based on the measure of fast EEG discharges at seizure onset has recently shown that these two structures are the most epileptogenic in patients with MTLE associated with normal MRI or HS.25 BFC decreases are concordant with a recent structural connectivity study conducted in a very large group of TLE patients, demonstrating bilateral decreased connectivity between the EC and the other mesial temporal structures as well as the neocortex.16 Such functional and structural connectivity alterations are likely to be linked to plasticity secondary to both generation and propagation of repetitive epileptiform discharges.30–32
It is noteworthy that the results of resting state fcMRI studies apparently contradict those based on EEG connectivity. Indeed, an increase in functional connectivity in the EZ has been measured from intracranial recordings.33 34 The mechanisms underlying these discrepancies are unknown to date and require further investigations. However, one of the main hypotheses remains a neurovascular decoupling in epileptic patients.34 Another hypothesis is that EEG connectivity and fcMRI capture different phenomena occurring on different timescale. Indeed, fcMRI is based on the cross-correlations between very slow fluctuations signals (<0.1 Hz), whereas EEG connectivity considers a broader frequency band but usually above 0.5 Hz (and up to 100–200 Hz).2 33 34 This may suggest complementary information from both modalities rather than a real discrepancy. Thus, in epileptic patients, functional connectivity based on slow fluctuations could be rather linked to the actual functional integrity of the macrosystems studied,6 14 whereas that based on faster fluctuations could be rather linked to abnormally high connectivity secondary to pathological electrophysiological changes.33–35
Increased basal functional connectivity is specific to the contralateral lobe to EZ
Because BFC increase is almost exclusively localised in the contralateral lobe to EZ, its detection permits indirect localisation of the EZ with a very high specificity. Moreover, BFC increases involved more specifically PostHip, AntHip and Amy. Increased functional connectivity of the hippocampus and Amy has already been described in patients with Alzheimer's disease compared with controls during a delayed match-to-sample face-recognition task.36 This increased connectivity was interpreted as reflecting an implicit signalling of emotional content to increase memory capacities. Although our resting state protocol did not allow assessment of connectivity behaviour specifically during this type of task, we hypothesise that BFC increases may reflect the same kind of compensatory mechanisms. In addition, it is now accepted that MTL not only plays a crucial role in declarative memory37 38 but also is implicated in WM.39–42 Altered WMQ in patients corroborate these findings. We observed altered WMQ in both left and right MTLE patients, and for both visual and auditory subtests. Moreover, the significant correlations between WMQ and increased BFC also support the hypothesis of MTL involvement in WM processes. However, our study was focused on an a priori network involving only the MTL structures and did not include extratemporal regions for statistical considerations (limited number of ROIs for multiple comparisons). It is thus possible that alterations of WMQ were also linked to wider functional network dysfunctions such as those involving the frontal lobe. Accordingly, Bernhardt et al found an increased structural connectivity between EC and homolateral orbitofrontal areas suggesting other compensatory mechanisms involving extratemporal areas.16 In addition, WMQ is a sensitive test frequently affected in neurological disorders despite its lack of specificity.43 This could also explain why, in this context, this cognitive test is found to correlate with BFC increases.
Diagnostic power of BFC to localise EZ
This study mainly shows that fcMRI is not able to directly determine the regions involved in seizure generation, since bilateral decrease in temporal lobe structures is the rule. However, BFC increases may be a reliable indicator of functional changes in the region contralateral to the EZ.
Currently, several studies have reported fcMRI data in drug-resistant partial epilepsies.20 44 45 However, to our knowledge, none have concentrated on individual results and on the potential clinical usefulness of the technique in the presurgical assessment of these epilepsies. The advantage of our study is that we were able to determine with a specificity of about 91% the EZ lateralisation. This method could represent an interesting clinical tool to help physicians.
We acknowledge that the majority of patients presented with HS, which is already a robust feature for EZ lateralisation. However, contralateral increased BFC was also found in seven of the nine patients with no HS. Our findings suggest that such BFC alterations represent a functional plasticity secondary to epileptic processes independent of the causal lesion. This is in accordance with the structural connectivity study by Bernhardt et al which found no significant relationship between TLE-related atrophy and EC connectivity.16 In addition, patients presenting with normal-appearing MRI also exhibited such contralateral increased BFC. Thus, we propose that resting-state fcMRI is a useful technique that could be added to the presurgical assessment of drug-resistant partial epilepsies.
Acknowledgments
The authors thank V Laguitton, for the neuropsychological assessment of patients, and A McGonigal, for the revision of the English version.
References
Footnotes
Funding This work was supported by CNRS, INSERM and ANR (CONNECTEPI). GB is the recipient of a PhD research grant delivered by the Region ‘Provence Alpes-Côte d'Azur’ and Deltamed.
Competing interests None.
Ethics approval Ethics approval was provided by the Local Ethics Committee of Marseille Public Hospital.
Provenance and peer review Not commissioned; externally peer reviewed.