Abstract
Summary: We describe imaging findings in a 2-year-old girl with neurocutaneous melanosis and malignant cerebral melanoma. Because the cerebral melanoma in this child was of the amelanotic type, high-signal intensity on unenhanced T1-weighted images was not present. The cutaneous lesions played a crucial role in establishing a correct (presumed) histopathologic diagnosis on the basis of the imaging findings. To our knowledge this is the first report describing an intracranial amelanotic malignant melanoma in association with neurocutaneous melanosis.
Case Report
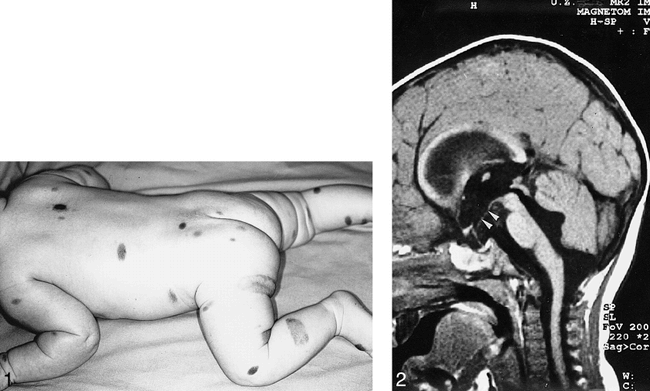
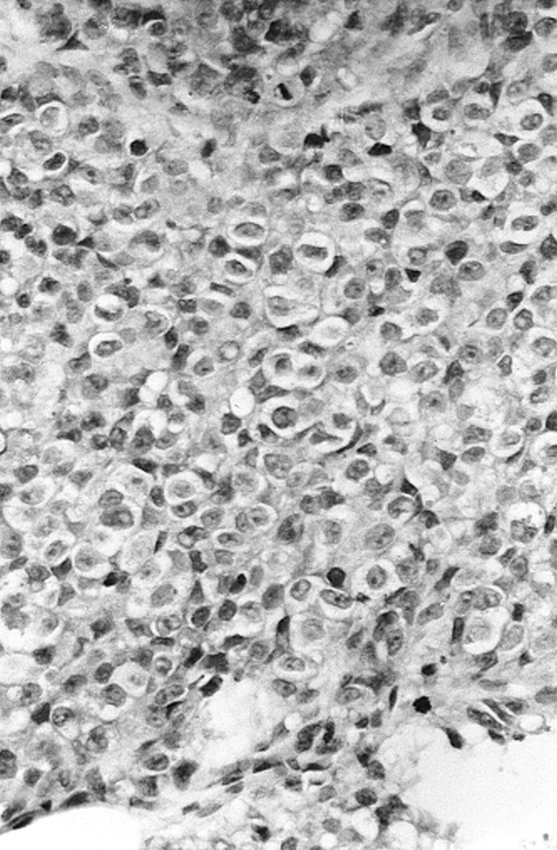
A girl with multiple congenital giant hairy nevi on her back, trunk, and head underwent resection of skin lesions 4 days after birth. Because the nevi recurred, they were resected again at the age of 1 year (Fig 1). At the age of 14 months she was admitted to our hospital with symptoms of somnolency, headache, and vomiting. Fundoscopy showed hyperemic optic disks with unsharp margins and dilated veins. An MR examination was performed on a Magnetom Impact 1.0 T (Siemens, Erlangen, Germany), with unenhanced 3-mm sagittal T1-weighted spin-echo (SE) images (500/12/3 [repetition time/echo time/excitations]). Flow in the aqueduct was evaluated with a fast SE T2-weighted sequence with 3-mm sagittal images (7000/112/3). The MR examination showed quadriventricular hydrocephalus without aqueduct stenosis (Fig 2). After ventricular drainage, symptoms resolved. Follow-up MR examination, performed 2 months later with unenhanced 5-mm axial and 3-mm sagittal T1-weighted SE images and 5-mm axial T2-weighted (3000/120/1) SE images, showed normal-sized ventricles. No tumor could be detected at the anterior aspect of the midbrain on the unenhanced T1-weighted images at this time. Because of changed CSF dynamics, tonsillar ectopia was present with slight downward herniation of the tonsils. Eight months later, the patient was readmitted to the hospital with ptosis of the right and intermittent upward deviation of the left eye. The patient was somnolent again. Consecutive unenhanced MR showed a right mesencephalic mass with isointense signal on T2-weighted SE images and hypointense signal on T1-weighted SE images, relative to the cerebral cortex. After intravenous administration of gadopentetate dimeglumine (0.1 mmol/kg body weight), strong and homogeneous enhancement was seen (Fig 3A–C). The patient underwent stereotactic biopsy. Histopathologic analysis of the specimen revealed a tumor with a diffuse and trabecular growth pattern, devoid of melanin pigment (Fig 4). Several mitoses and brain invasion suggested a malignant tumor. Immunohistochemistry showed a strong positive reaction for HMB 45 and S 100 in tumor cells, consistent with the diagnosis of amelanotic malignant melanoma. Additional stainings for lymphoma, germinoma, and glial tumors were negative. The patient died 1 month later from intratumoral bleeding and brain stem compression. Autopsy was refused by the parents.
Multiple congenital hairy nevi are seen on a 6-month-old.
fig 2. T1-weighted (500/12/2—repetition time/echo time/excitations) midsagittal image shows hydrocephalus with enlarged lateral, third, and fourth ventricles in a 14-month-old. Note the downward bending of the floor of the third ventricle (arrowheads).
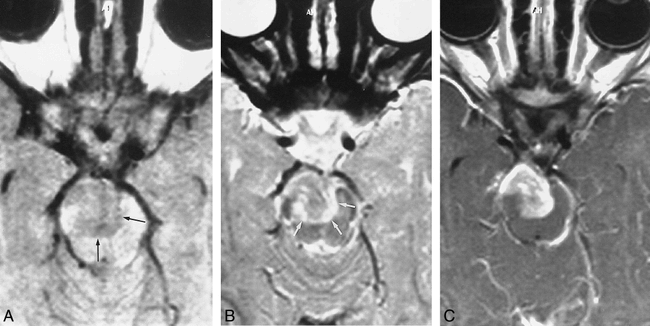
Follow-up MR examination of 2-year-old girl.
A, An unenhanced axial T1-weighted (500/12/3) image shows a hypointense lesion anterior and superior to the pons with invasion of the mesencephalon (black arrows).
B, Axial T2-weighted (3000/120/1) image shows the mass is isointense with surrounding edema (white arrows) relative to the cerebral cortex.
C, Note the strong enhancement after gadolinium administration.
Routine hematoxylin and eosin staining is shown. The nuclei are prominent and have a finely distributed chromatin with a large nucleolus. Tumor cells have a clear cytoplasm, devoid of melanin pigment
Discussion
Neurocutaneous melanosis is a rare phakomatosis characterized by pigmented nevi that are large or multiple or both, and leptomeningeal melanosis or melanoma, without evidence of malignancy in the skin lesions, and without involvement of nonmeningeal organs (1). Most cases are sporadic, and no predisposition according to sex is noted (2). Fox proposed several diagnostic criteria: the presence of large or multiple congenital melanocytic nevi (one of which is at least 20-cm in diameter) with benign (melanosis) or malignant (melanoma) CNS tumors; absent melanomatous malignant involvement of the skin; and no evidence of malignant melanoma in any organ outside the CNS (1). The disease may be associated with another neurocutaneous syndrome such as Sturge-Weber or von Recklinghausen's disease (3). Associations were also reported with the Dandy-Walker complex, spinal lipoma, and arachnoid cyst (4, 5).
Histopathologic examination shows accumulation of melanotic cells in the basal arachnoidea and pia mater of the ventral surface of the mesencephalon, pons, medulla and cerebellum, and the upper cervical spinal and lumbosacral cord, resulting in leptomeningeal melanosis (1). Presence of melanocytosis in CSF makes leptomeningeal involvement likely (6). Parenchymal melanin deposits probably represent melanocytes in the perivascular spaces. The anterior temporal lobes, and particularly the amygdala, seem to be the most frequent locations for parenchymal melanocytic accumulation. Other preferential sites include the cerebellum, thalami, and the base of the frontal lobe (4, 7).
Clinically, CNS involvement is suspected by the occurence of lethargy, seizures, and vomiting, signs suggestive of raised intracranial pressure from hydrocephalus. Cranial nerve palsy is associated frequently. Subdural or parenchymal hemorrhage or both, syringomyelia, and spinal arachnoiditis can complicate the clinical presentation (2). Hydrocephalus occurs in the majority of patients, because of meningeal thickening from CSF outflow obstruction or decreased CSF resorption (2, 4).
The imaging clue to the diagnosis of leptomeningeal melanosis or parenchymal melanin deposits is T1 shortening on MR. This is often ascribed to the paramagnetic metal scavenging of melanoma cells resulting in a spontaneous high signal of melanin on T1-weighted images. Other authors attribute T1 shortening to paramagnetic free radicals known to occur in melanin (8). Leptomeningeal enhancement in cases of absent T1 shortening also indicates leptomeningeal melanosis (9). According to Barkovich, the absence of meningeal enhancement does not exclude the diagnosis of neurocutaneous melanosis (7). In our patient, T1 shortening along the leptomeningeal structures or in the brain parenchyma was never noted. On the other hand, a hypointense mass was seen in the mesencephalon on the unenhanced T1-weighted images. This finding, combined with the intermediate signal of the tumor on the T2-weighted images and strong and homogeneous enhancement on the T1-weighted images after gadolinium administration, is nonspecific. In view of the multiple melanotic cutaneous manifestations found in our patient, the presumptive diagnosis of a primary amelanotic melanoma of the brain could be suggested.
Byrd et al (9) did not observe T1 shortening in the leptomeninges on unenhanced MR images, and attributed this to the number and maturity of the melanocytes. Strong enhancement of the leptomeninges was seen in all five cases presented. Massive melanin pigment was present in one of five cases in which the disease was reported. According to Isiklar et al (10) only the minority of melanoma metastases have the anticipated MR pattern of T1 shortening. The majority of tumors that exhibit this melanotic pattern have more than 10% of melanin-containing cells. The putative MR pattern for amelanotic melanoma is nonspecific, as over half of tumors with this pattern contain melanin, but always in less than 10% of cells (10). In our patient, the tumor was devoid of melanin pigment on histologic examination.
There are reports of MR-revealed neurocutaneous melanosis without detectable leptomeningeal melanosis but with temporal lobe malignant melanoma (4, 11). Eaves et al suggested that primary brain melanoma without diffuse leptomeningeal involvement in patients with giant intradermal nevi is a variant of neurocutaneous melanosis (12). No foci of leptomeningeal melanosis could be detected in our patient. The development of hydrocephalus, however, makes leptomeningeal involvement probable. No contrast was administered in our patient before the mesencephalic tumor occurred.
Differentiation between benign and malignant parenchymal melanocytosis on MR is difficult. Necrosis, edema, and hemorrhage are suggestive of malignancy, but can be absent in malignant melanomas (7, 11). Leptomeningeal enhancement is not pathognomonic for leptomeningeal melanosis. It can be seen in infectious and metastatic disease and in cases of spreading of a primary brain tumor. It is not possible to differentiate benign from malignant leptomeningeal melanosis with MR imaging (9). Follow-up with serial neurologic examination and imaging studies is advised. Surgical intervention must be considered only if evidence of progression is documented (7). Prognosis is poor once CNS symptoms occur.
About 100 cases of neurocutaneous melanosis have been reported. To our knowledge this is the first report of neurocutaneous melanosis associated with intracranial amelanotic malignant melanoma. This case report illustrates how the presence of cutaneous lesions can be helpful in the interpretation of intracerebral lesions. This is well known for other more frequent phakomatoses such as neurofibromatosis or Sturge-Weber disease, and turned out to be particularly true in this neurocutaneous syndrome.
Footnotes
↵1 Address reprint requests to Bart Vanzieleghem, University Hospital Gent, Department of Radiology, De Pintelaan 185, 9000 Gent, Belgium.
References
- Received March 4, 1998.
- Accepted after revision August 25, 1998.
- Copyright © American Society of Neuroradiology