Abstract
BACKGROUND AND PURPOSE: Diffusion and perfusion MR imaging have been reported to be valuable in the diagnosis of acute ischemia. Our purpose was to ascertain the value of these techniques in the prediction of ischemic injury and estimation of infarction size, as determined on follow-up examinations.
METHODS: We studied 18 patients with acute ischemic stroke who underwent echo-planar perfusion and diffusion imaging within 72 hours of symptom onset. Quantitative volume measurements of ischemic lesions were derived from relative mean transit time (rMTT) maps, relative cerebral blood volume (rCBV) maps, and/or apparent diffusion coefficient (ADC) maps. Follow-up examinations were performed to verify clinical suspicion of infarction and to calculate the true infarction size.
RESULTS: Twenty-five ischemic lesions were detected during the acute phase, and 14 of these were confirmed as infarcts on follow-up images. Both ADC and rMTT maps had a higher sensitivity (86%) than the rCBV map (79%), and the rCBV map had the highest specificity (91%) for detection of infarction as judged on follow-up images. The rMTT and ADC maps tended to overestimate infarction size (by 282% and 182%, respectively), whereas the rCBV map appeared to be more precise (117%). Significant differences were found between ADC and rMTT maps, and between rCBV and rMTT maps.
CONCLUSION: Our data indicate that all three techniques are sensitive in detecting early ischemic injury within 72 hours of symptom onset but tend to overestimate the true infarction size. The best methods for detecting ischemic injury and for estimating infarction size appear to be the ADC map and the rCBV map, respectively, and the diffusion abnormality may indicate early changes of both reversible and irreversible ischemia.
The ability to distinguish reversible from irreversible ischemia, to predict which tissues will evolve to irreversible damage unless therapy is undertaken, and to estimate infarction size is important for the management of patients with acute stroke. Recent advances in MR imaging techniques provide new information directly related to the underlying pathophysiology at the cellular and microscopic levels and may allow the diagnosis of acute ischemia and estimation of infarction size. Diffusion imaging reflects abnormal water movement in acute ischemic tissues caused by the failure of the high-energy Na-ATP pump and is sensitive to hyperacute ischemia within a few minutes after onset (1). In both animal models and clinical cases, early ischemic changes have been reported on diffusion images when standard T2-weighted images showed no abnormality (2–4). Perfusion imaging, including relative mean transit time (rMTT) and relative cerebral blood volume (rCBV) maps, provides information related to the status of cerebral blood flow (CBF) to the brain parenchyma (5).
Both diffusion imaging, including apparent diffusion coefficient (ADC) maps, and perfusion imaging have been reported to be superior to conventional MR imaging and/or CT in the detection of acute ischemia (6). The combination of diffusion and perfusion imaging can be helpful in identifying tissue viability in acute ischemia (6); however, few studies have compared diffusion and perfusion abnormalities in acute ischemia. In addition, studies evaluating the usefulness of perfusion imaging in the prediction of acute ischemic injury and the estimation of the true infarction size remain controversial (6–8). The purpose of this study was to determine if ADC, rMTT, and rCBV maps obtained within 72 hours of symptom onset of acute ischemic stroke were useful in the prediction of irreversible ischemic injury (infarction) and the estimation of the extent of involvement (infarction size).
Methods
We studied 18 patients admitted to our institution with a clinical diagnosis of acute ischemic stroke who underwent perfusion and diffusion MR imaging. The group included 10 men and eight women, ranging in age from 27 to 80 years (mean age ± SD, 56 ± 17 years). Inclusion criteria for the study were as follows: 1) patients had apparent and acute neurologic deficits corresponding to abnormal MR findings, 2) the MR imaging study was performed within 72 hours of symptom onset, 3) follow-up MR or CT studies were performed 2 weeks or more after symptom onset to verify the clinical suspicion of infarction and to estimate the true infarction size, and 4) no known clinical events occurred between the initial and follow-up studies. On the basis of symptoms, onset pattern, radiologic findings, and results of other examinations, the cause of ischemic stroke in these patients was classified as atherothrombotic infarction (n = 11), cardioembolic infarction (n = 5), or cerebral vasculitis (n = 2). Patients received standard treatment for acute ischemic stroke; either anticoagulation or antiplatelet therapy. No patient received thrombolytic or neuroprotective therapy.
MR imaging was performed on a 1.5-T unit with echo-planar imaging capability. Before perfusion and diffusion MR imaging, axial T1- and T2-weighted standard spin-echo sequences were obtained. Imaging parameters were 600/20/1–2 (TR/TE/excitations) for T1-weighted sequences and 2000–2500/30 for T2-weighted double-echo sequences. Section thickness was 5 mm, matrix was 256 × 192, and field of view (FOV) was 24 cm.
Dynamic echo-planar spin-echo contrast-enhanced images (2000/100/1, 40 × 20 FOV, 256 × 128 matrix, one shot) were obtained in eight to 10 axial sections at 2-second intervals for 60 seconds immediately after an intravenous bolus injection of 0.1 mmol/kg gadopentetate dimeglumine was administered at 9 mL/s with an MR-compatible power injector. Qualitative perfusion maps were calculated for rMTT and rCBV. Details of the theory of the rMTT and rCBV calculations in echo-planar perfusion imaging have been described previously (9). Diffusion images were obtained using echo-planar imaging (4000/125/1, 40 × 20 FOV, and 256 × 128 matrix). Diffusion gradients were applied independently on each axis of the magnet. Nine diffusion-weighted images were obtained along each axis (bxi, byi, bzi, i = 1.9) ranging from b values of 0 to 1663 s/mm2 with linear steps of the b value. Anisotropic diffusion coefficient images were calculated for each direction of the diffusion gradient using the relationship 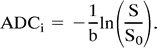 The anisotropic diffusion maps were then combined to form an isotropic diffusion map by computing the average diffusion coefficient
The anisotropic diffusion maps were then combined to form an isotropic diffusion map by computing the average diffusion coefficient 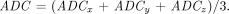 Our in-house software generated the rMTT, rCBV, and ADC maps automatically in fewer than 4 minutes for 11 sections using these techniques. The maps were scaled and saved in an image format that could be transferred to the scanner and filmed for the radiologist to review. Total time for the acquisition and processing of these images was about 10 minutes.
Our in-house software generated the rMTT, rCBV, and ADC maps automatically in fewer than 4 minutes for 11 sections using these techniques. The maps were scaled and saved in an image format that could be transferred to the scanner and filmed for the radiologist to review. Total time for the acquisition and processing of these images was about 10 minutes.
These ADC, rMTT, and rCBV maps were assessed qualitatively by five neuroradiologists. The abnormality was outlined on each image by two neuroradiologists, who were blinded to the date and time of image acquisition. Each neuroradiologist determined the volumes independently, and the values were averaged. These were confirmed or corrected in each section independently by a consensus of three other neuroradiologists. Each abnormality depicted by any of these maps was considered a separate acute ischemic lesion. These abnormalities depicted by diffusion and perfusion imaging during the acute phase of stroke were then correlated with clinical symptoms to determine whether they qualified as acute ischemic lesions. Follow-up MR imaging or CT was obtained from 2 weeks to 16 months later (mean, 8 months) to determine if infarction had occurred and to assess the prediction rate of the ADC, rMTT, and rCBV maps for identifying irreversible tissue damage. Quantitative volume measurements of acute ischemic lesions shown on rMTT, rCBV, ADC maps, and follow-up images were also performed on the workstation. The quantitative analysis of lesion size determined by each imaging technique was compared with and normalized to the true infarction size as determined from the follow-up studies. For statistical analysis, the differences among the three types of maps were assessed with ANOVA. The differences were considered to be significant at P < .05.
Results
The imaging time during acute ischemia ranged from 6 to 72 hours (mean, 58 hours). A total of 25 acute ischemic lesions were identified by diffusion and/or perfusion imaging. Fourteen (56%) of the 25 acute ischemic lesions were confirmed as infarctions on follow-up studies.
Prediction of Acute Ischemic Injury
The detection rate for each technique is summarized in Table 1. The sensitivity of the ADC, rMTT, and rCBV maps was 85.7%, 85.7%, and 78.6%, respectively, and there was no significant difference among the three techniques. The specificity of the ADC, rMTT, and rCBV maps was 63.6%, 63.6%, and 90.9%, respectively, and there were significant differences between the rCBV maps and the ADC and rMTT maps (P < .05). All three techniques were statistically equal in the prediction of ischemia, whereas the rCBV map was the most specific for the prediction of infarction. In this series, four (25%) of 16 ADC abnormalities were false positives or reversible ischemia. (Note that in Table 1, the patients without infarction on follow-up images who had either positive ADC maps or positive rMTT maps are different patients.)
Detection rates for each MR imaging technique
Estimation of Infarction Size
The quantitative volume measurement of the acute ischemic lesions, as identified by each imaging technique, and the infarction size, as determined by follow-up studies, are shown in Table 2. All three techniques overestimated the infarction size. Both rMTT and ADC maps tended to overestimate greatly the true size of the infarction (by 282% and 182%, respectively), whereas the rCBV map appeared to be more precise (117%). There were significant differences between the ADC and rMTT maps, and between the rCBV and rMTT maps (P < .05).
Infarction size estimated by each MR imaging technique
Illustrative Cases
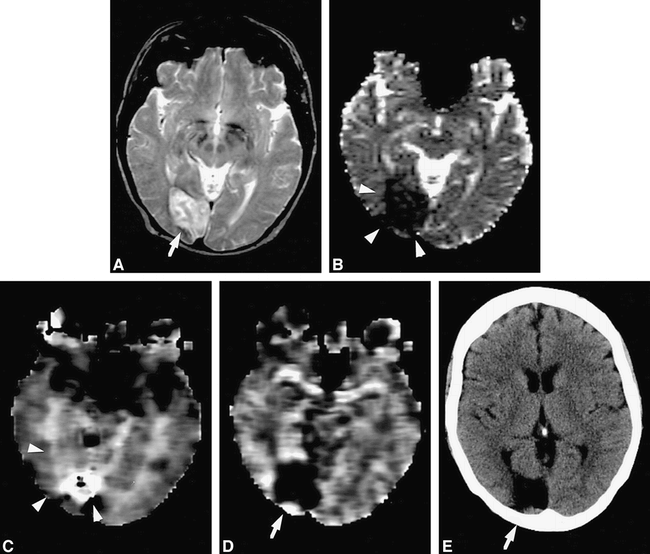
Case 1 (Fig 1)
Case 1: 61-year-old woman who had imaging 48 hours after the onset of symptoms.
A, T2-weighted MR image shows a high intensity area (arrow) in the right occipital region.
B, The ADC map shows an abnormal occipital lesion (arrowheads), which is much larger than that seen on initial T2-weighted image (A) and follow-up CT scan (E).
C, The rMTT map also shows an abnormal occipital lesion (arrowheads), which is larger than that seen on initial T2-weighted image (A) and follow-up CT scan (E).
D, The rCBV map shows an abnormal occipital lesion (arrow), which is a little larger than that seen on T2-weighted image (A) but almost the same size as that on follow-up CT scan (E).
E, Follow-up CT scan 11 months later shows infarction (arrow) in the right occipital region.
A 61-year-old woman was admitted with left homonymous hemianopsia. She had a recent history of hypertension and diabetes mellitus. MR imaging was performed 2 days after symptom onset. T2-weighted images showed a high-intensity area in the right occipital region. The lesions depicted on ADC and rMTT maps were much larger than those depicted on T2-weighted images and rCBV maps. On follow-up CT studies, obtained 11 months later, ADC and rMTT maps overestimated infarction size (by 140% and 190%, respectively), whereas rCBV maps had the most precise estimation of infarction size (110%) (Table 2, lesion 7).
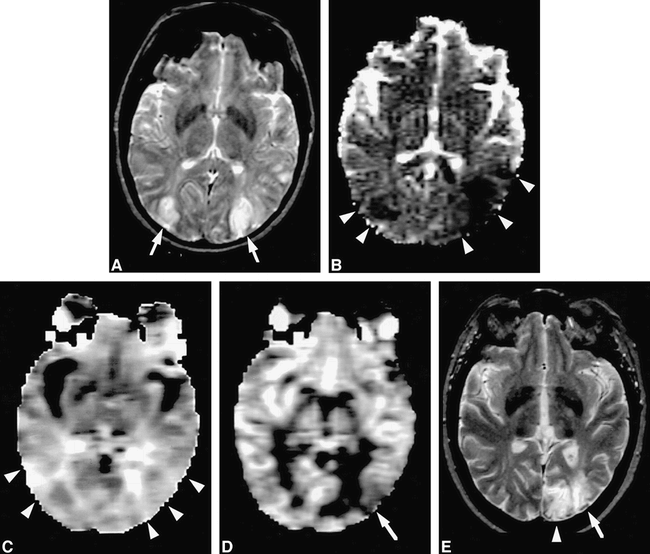
Case 2 (Fig 2)
Case 2: 35-year-old woman who had imaging 72 hours after the onset of symptoms.
A, T2-weighted MR image shows bilateral posterior watershed lesions (arrows).
B, The ADC map also shows bilateral posterior watershed lesions (arrowheads), but both lesions are larger than those seen on T2-weighted images.
C, The rMTT map shows mild hypoperfusion extensively at the territories of both posterior cerebral arteries (arrowheads).
D, The rCBV map shows only a left-sided lesion (arrow), including posterior watershed and part of the posterior cerebral artery territory.
E, The 16-month follow-up T2-weighted image confirmed small left posterior watershed infarction (arrow) and another infarction at the territory of the left posterior cerebral artery (arrowhead), which was not apparent on initial T2-weighted image or ADC map but was visible on rCBV map.
A 35-year-old woman was admitted for a disturbance of consciousness. She had multiple infarctions related to cerebral vasculitis. MR imaging was performed 3 days after a recent event, which was accompanied by progressive and persistent symptoms. T2-weighted images showed bilateral posterior watershed lesions. ADC maps showed similar findings, but the lesions were larger than those depicted on T2-weighted images on the left side. The rMTT map showed mild hypoperfusion extensively at the territories of both posterior cerebral arteries; however, the rCBV map showed only a left-sided lesion, including the posterior watershed and part of the territory of the posterior cerebral artery, which was much smaller than that shown on the ADC map. The right watershed distribution was normal. The 16-month follow-up T2-weighted MR study confirmed left watershed infarction and left posterior cerebral artery infarction, which was not shown on the initial T2-weighted image or the ADC map, but was apparent on the rCBV map, and there was no right watershed infarction. In this case, ADC and rMTT maps overestimated the infarction area, whereas the rCBV map was close to the true size of the infarction area.
Discussion
At present, only intravenous thrombolysis using tissue plasminogen activator within 3 hours has been shown to be beneficial for the treatment of acute ischemic stroke (10). Rapid assessment and early intervention are required to improve patient outcome. The therapeutic window is thought to be limited to the first few hours after symptom onset, and diagnostic imaging that predicts the outcome of acute ischemic injury and estimates the size of the irreversibly damaged tissue is needed. Each new neuroimaging technique provides a different type of information and sensitivity, reflecting various degrees of ischemic injury caused by decreased CBF and altered metabolic state. In this study, we attempted to assess the efficacy of diffusion and perfusion MR imaging in acute ischemic stroke in the prediction of irreversible ischemic injury and estimation of the extent of involvement.
In animal studies, the signal abnormality in diffusion imaging appears a few minutes after occlusion of the middle cerebral artery and enlarges over the next 24 hours (2, 11, 12). The abnormality in diffusion imaging is equivalent to that on T2-weighted images by 24 hours after the onset of ischemia (12–14); however, cytotoxic and vasogenic edema may cause an overestimation of lesion volume. Baird and Warach (15) suggested that there is no absolute threshold of ADC decrease that predicts evolution to infarction, because time and ADC decrease do not necessarily imply evolution to infarction.
In clinical studies, diffusion imaging also has been reported to have a high sensitivity and specificity for acute ischemia (16–18); however, the pathophysiologic changes in acute ischemia in humans are likely to be more heterogeneous than those found in animal models. There are reported to be two phases in the time course of ADC changes: a significant reduction at 96 hours from stroke onset and an increasing trend from reduction to pseudonormalization to elevation at later, subacute to chronic, time points (>7 days) (19). Furthermore, Baird et al (20) indicated that enlargement of human cerebral ischemic lesions as measured by diffusion imaging occurred in 43% of patients studied within the first 53 hours. Similarly, Sorensen et al (6) showed that the early diffusion imaging abnormality (11 hours or less) underestimated the infarct volume depicted on later imaging studies (mean, 5.5 days). It remains to be determined whether their underestimation is due to initial imaging obtained too early or to follow-up imaging obtained too soon. We agree that persisting hypoperfusion, including an ischemic penumbra, may play a role in the enlargement of the ischemic lesion due to elevation of the infarction threshold: a time-dependent parameter. The results of our previous report, in which we found that the therapeutic window of tissue viability could be longer than 6 hours or more in patients who had enough residual CBF in the ischemic lesion, may support the speculation of an ischemic penumbra (21). In addition, other processes, such as delayed neuronal cell death or apoptosis, may also play a role in the progressive enlargement of infarction size. Du et al (22) found that brain infarction after mild transient focal ischemia in rats can develop in a delayed fashion more than 3 days after the original insult. On the other hand, the ischemic lesion volume and ADC values as measured by diffusion imaging may be potential parameters for predicting clinical outcome in patients with acute stroke (23, 24).
Although T2-weighted images generally indicate the irreversible damage of acute ischemia, the abnormal findings of diffusion imaging may consist of early changes of both reversible and irreversible ischemia (25). The diffusion abnormality in global ischemia is reversible by early reperfusion within 12 minutes (26). Regression of the diffusion abnormality was also reported in reperfusion models (27, 28). Miyabe et al (29) indicated that if reperfusion occurred before the ADC value decreased to less than approximately 70% of the control value for 10 to 20 minutes, ADC changes were usually reversible. A few authors have reported the regression of the diffusion abnormality in humans (4, 6). The present study suggests that diffusion imaging had the highest sensitivity but was not as specific as the rCBV map in predicting acute ischemic injury and tended to overestimate infarction size in patients studied within 72 hours of stroke onset. In addition, in this limited sample, four (25%) of 16 ADC abnormalities were false-positives or reversible ischemia. These results may support the contention that the diffusion abnormality indicates early changes of both reversible and irreversible ischemia.
Perfusion imaging provides direct information related to a reduction in blood flow, which reflects the primary underlying pathophysiology of acute ischemia. Although perfusion imaging cannot produce absolute values but only semiquantitative data in estimating rMTT and rCBV maps, it can be performed quickly, which is essential for the emergency management of patients with acute ischemic stroke. Furthermore, higher temporal resolution and multisection images can be achieved by echo-planar imaging techniques. Our previous study indicated that perfusion imaging was superior to diffusion imaging in the assessment of hemodynamic changes in symptomatic patients with severe occlusive carotid artery disease (9). In another study, we reported that ischemic tissue with prolonged rMTT and a marked decrease in rCBV tends to suffer irreversible damage (5). A mild decrease in rCBV with prolonged rMTT may suggest an area of reversible ischemia. A marked increase in rCBV may show the state of luxury perfusion in subacute ischemia. Moreover, Röther et al (30) suggested that it was possible to differentiate severely ischemic tissue from periinfarct parenchyma by rCBV maps in hyperacute ischemia.
The mismatch between diffusion and perfusion imaging in patients with acute ischemic stroke has been reported in several studies. Sorensen et al (6) indicated that in patients studied within 10 hours of onset, the abnormality in rMTT maps was larger than that in rCBV maps and on diffusion images. Rordorf et al (8) demonstrated that diffusion lesion volumes were smaller than the volumes of rCBV map abnormalities in patients studied within 12 hours of onset, and the early CBV abnormality was slightly better than the diffusion abnormality as a predictor of final infarction size. Our results are similar to the reported findings in which the rMTT map overestimated the final infarction volume and the rCBV map provided the best estimation of lesion volume; however, they are different in that, in their study (6, 8), diffusion imaging underestimated volume whereas, in ours, it overestimated the size of the infarction. The size of the abnormality in diffusion and perfusion imaging depends on the imaging time from the onset of symptoms.
On the other hand, Barber et al (31) suggested that diffusion and perfusion (only rMTT map) imaging may permit the selection of rational therapeutic strategies based on the presence or absence of potentially salvageable ischemic tissue. The rMTT map is markedly sensitive to the hemodynamic compromise at the chronic hypoperfused stage as well as to the sudden changes of perfusion status in acute ischemia (5). Furthermore, the CBF threshold for positive findings in both diffusion imaging and rMTT maps may be higher than the infarction threshold. Qualitative assessment of diffusion imaging and rMTT maps alone may not be helpful in the selection of patients for thrombolytic therapy. To improve patient selection for thrombolytic therapy, we think it may be necessary to assess tissue viability or reversibility more quantitatively by diffusion and perfusion imaging, including both rMTT and rCBV maps, just as we used single-photon emission CT data in a recently reported study (21).
These results concerning sensitivity, specificity, and estimation of infarction size by each technique differ from those in previous reports (6, 16–18, 24). One possible reason is the time of imaging from stroke onset. Our mean imaging time was 58 hours from symptom onset, by which time lesions might have amassed extensive vasogenic edema. Particularly in our case of vasculitis (Fig 2), the changes on T2-weighted images, as well as the diffusion abnormality, were no longer evident on follow-up studies. Another possibility is related to the difference in detecting various degrees of cerebral hypoperfusion (CBF thresholds) by different imaging techniques. Each method may have a different sensitivity in detecting a decrease in CBF from normal range. The CBF threshold for positive findings may vary with each technique, and it appears to be higher than the infarction threshold based on the limited data generated by our techniques. For instance, in one animal study (32), diffusion-weighted imaging findings became abnormal when CBF dropped to 34 to 41 mL/100 g per minute after middle cerebral artery occlusion. These values are much higher than that of the so-called threshold for energy failure (below 10 mL/100 g per minute) and of the ischemic penumbra (10–20 mL/100 g per minute) (33). The other possibility is the variation in imaging techniques used, including the scanner, pulse sequences, injection rate of contrast agent, methods of deriving the parameters, and generation of maps.
We realize that our study has several shortcomings. First, patients in whom hyperacute stroke (less than 6 hours from symptom onset) was diagnosed were not included. Second, the time of the follow-up study varied. The true infarction size may have been over- or underestimated in some cases because of vasogenic edema in the early stage or atrophy in the chronic stage. Third, although diffusion-weighted imaging is the most commonly used sequence in addition to routine MR imaging, diffusion-weighted imaging was not evaluated in this study, because the ADC map is usually used in our institution to assess ischemia quantitatively. Finally, there might or might not have been some patients in whom spontaneous reperfusion occurred, and reperfusion may influence analysis of perfusion data.
Conclusion
Diffusion and perfusion imaging, including ADC, rCBV, and rMTT maps, were sensitive in the detection of acute ischemia within 72 hours of symptom onset but tended to overestimate the true infarction size. On the basis of our data, we think the best methods for detecting ischemic injury and for estimating infarction size are the ADC and rCBV maps, respectively, and that the diffusion abnormality may indicate early changes of both reversible and irreversible ischemia. Because these techniques provide different pathologic information, the optimal way to diagnose acute ischemia and to estimate infarction size is to use these imaging techniques at any given time from stroke onset and to integrate all the information they provide.
Acknowledgments
We gratefully acknowledge Phyllis Bergman for her expert assistance in the preparation of this manuscript.
Footnotes
References
- Received October 26, 1998.
- Accepted after revision February 17, 1999.
- Copyright © American Society of Neuroradiology