Abstract
BACKGROUND AND PURPOSE: The safe performance of percutaneous transluminal cerebral angioplasty for intracranial atherosclerotic lesions requires that the risk of complications, such as acute occlusion or symptomatic dissection, and restenosis be reduced. Our purpose was to assess the effectiveness, safety, and short-term arteriographic and clinical outcome of cerebral angioplasty and stenting (CAS) for intracranial vertebrobasilar and distal internal carotid atherosclerotic occlusive lesions.
METHODS: Between March 1998 and November 1998, 10 patients with 12 intracranial atherosclerotic lesions of the vertebrobasilar artery and the distal internal carotid artery underwent treatment with flexible balloon-expandable coronary stents.
RESULTS: Although in two of the 10 patients CAS was not successful because of the inability to access the site of arterial stenosis, 10 lesions in eight patients were successfully dilated with stents. No complications occurred during or after the procedure and no neurologic ischemic events or restenoses occurred during the follow-up period.
CONCLUSION: CAS appears to be a safe and effective means for treating intracranial atherosclerotic occlusive disease, yielding a favorable arteriographic and clinical outcome.
Percutaneous transluminal cerebral balloon angioplasty (PTCBA) has been used to treat stenosis of the intracranial artery since 1980, when Sundt et al (1) performed the first intracranial angioplasty in the basilar artery (BA). Although some investigators have reported good results after intracranial PTCBA (2–4), others have described high rates of morbidity and mortality (5, 6). The lack of devices to resolve untoward complications associated with intracranial PTCBA has undoubtedly contributed to these statistics; indeed, the development of several types of balloon-expandable metallic stents for treating the coronary artery has reduced the occurrence of procedure-related morbidity (7, 8). Long-term patency after coronary stenting has also been reported (9). The purpose of this study was to assess the effectiveness, safety, and short-term arteriographic and clinical outcome of treatment with flexible coronary stents for intracranial atherosclerotic occlusive lesions.
Methods
A total of 93 consecutive percutaneous transluminal cerebral angioplasties of the intracranial artery were performed in our institution between January 1992 and November 1998. Among 19 consecutive cerebral angioplasties performed in 17 patients between March 1998 and November 1998, 12 intracranial atherosclerotic lesions of the vertebrobasilar artery and the distal internal carotid artery (ICA) in 10 patients were treated with cerebral angioplasty and stenting (CAS), whereas seven lesions of the middle cerebral artery (MCA) in seven patients were treated with balloon angioplasty alone. The mean age of the 10 patients (nine men and one woman) was 68 ± 9 years. Lesions involved the distal ICA in four patients, the intracranial vertebral artery (IVA) in five patients, and the BA in three patients (Table). Nine of the 10 patients were symptomatic and one was asymptomatic.
Patients' characteristics
Inclusion criteria for the CAS study were 1) significant stenosis (60% or more) of one of the following major intracranial arteries: the distal ICA, the IVA, and the BA; 2) crescendo transient ischemic attacks unresponsive to maximal medical management by intravenous heparin or antiplatelet medications, a minor stroke with persistence of symptoms or signs in the distribution of the stenotic artery, or an acute stroke caused by high-grade stenosis or total occlusion of the major intracranial arteries described above; 3) presence of symptoms within 6 months prior to treatment; or 4) a need of vascular reconstruction before general anesthesia (symptomatic or asymptomatic). Exclusion criteria were 1) severe neurologic deficit from a major stroke; 2) chronic total occlusion of more than 6 months' duration; 3) chronic total occlusion greater than 10 mm in length; 4) chronic total occlusion without a visible distal segment on angiograms; and 5) stenosis or occlusion of the MCA, the anterior cerebral artery, or the posterior cerebral artery.
Before CAS, all patients received a complete neurologic examination, routine laboratory testing, and brain CT or MR imaging to evaluate the cerebral lesions. All patients except one (case 1) received 200 mg of ticlopidine per day at least 3 days before and after successful CAS. Nine patients underwent elective CAS; one patient (case 1) was treated acutely.
Employing the criteria developed by the North American Symptomatic Carotid Endarterectomy Trial (10, 11), we calculated the stenosis rate on angiograms before and immediately after CAS and at follow-up to compare the diameter of the vessel at the site of greatest narrowing with the diameter of a normal artery distal to the lesion. When a stenosis rate of 60% or greater before treatment was decreased to less than 50% immediately after CAS, the procedure was considered arteriographically successful. Clinical success was defined as arteriographic success with no major complications, such as in-hospital mortality, stroke, or emergency EC/IC bypass surgery. Neurologic signs and symptoms during balloon inflation were not regarded as complications.
Written informed consent for CAS and treatment during follow-up was obtained from all patients or their families. CAS was performed with the patient under local anesthesia via a femoral arterial route. Dextran 40 (10% w/v low-molecular-weight dextran) was started at 1000 to 2000 mL/day 1 hour before CAS and was continued until the next day. A 6F to 7F guiding catheter (MR2, Medikit, Tokyo, Japan) was selectively placed via a femoral arterial route into the ICA or the vertebral artery. Heparin (10,000 U) was injected intravenously to avoid thrombus formation during the procedure, and isosorbide dinitrate (1.25 to 2.50 mg) was injected as a single rapid bolus into the vertebral artery or the ICA through a guiding catheter to prevent balloon catheter–induced vasospasm. A 0.014-inch guidewire (Dasher-14, Boston Scientific/Target Therapeutics, Fremont, CA) was navigated across the stenosis or through the occlusion. A very flexible 20-mm-long balloon catheter (Ranger, Boston Scientific/SciMed, Maple Grove, MN) was guided over the wire and then inflated twice at 6 atm for 60 seconds to predilate the lesion. The balloon catheter was replaced by a flexible balloon-expandable coronary stent using the 0.014-inch 300-cm exchange guidewire (Taper-14, Boston Scientific/Target Therapeutics). The gfx coronary stent (Medtronics, Santa Rosa, CA) was used in eight vertebrobasilar arteries and in two distal ICA lesions, and the Multilink coronary stent (Guidant/Vascular Intervention, CA) was used in two distal ICA lesions. The gfx stent was deployed at 9 atm for 15 seconds, and the Multilink stent at 6 atm for 15 seconds. If dilatation of the lesions was not adequate, stents were further expanded with balloon catheters. Since April 1998, we have used the Multilink stent for distal ICA lesions, because its frame seems to better support the carotid wall.
Before CAS, all patients were examined neurologically; afterward, they were monitored every month at an outpatient clinic, where neurologic status was assessed. The patients with clinically successful CAS underwent arteriographic follow-up 3 months later. Arteriographic restenosis was defined as occlusion of 50% or more of the diameter of the vessel at the follow-up examination.
The length of a lesion and the diameter of the vessel at the referential or stenotic site were measured on arteriograms obtained with the lesion positioned at the isocenter. Because images obtained with digital subtraction angiography in our institution showed lesions to be 1.4 times larger at the isocenter, a 14-mm scale was placed on an image-intensifier entrance plane with a grid to indicate a 10-mm scale on the angiograms.
Continuous variables were expressed as mean ± 1 SD. Stenosis rates before and after stenting and at follow-up were compared by using a paired Student's t-test. A probability of less than P = .05 was considered statistically significant. StatView 4.5 statistics software was used.
Results
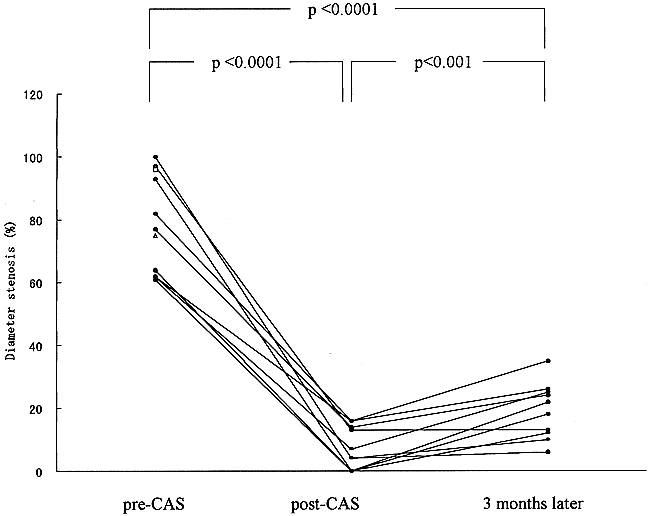
The baseline angiogram showed a pre-CAS stenosis of 81 ± 16% in 12 lesions. A coronary stent was implanted easily in 10 of the 12 lesions, and eight of the 10 patients were treated successfully with stenting. In the 10 lesions successfully treated, a pre-CAS stenosis of 80 ± 17% was reduced to 7 ± 7% (P < .0001) on the immediate post-CAS angiogram (Figs 1–3). In one patient (case 6), the expansion of a 3.0-mm gfx stent implanted in the left IVA was inadequate, and a 3.5-mm Ranger balloon was used to further expand both the stent and the lesion. In another patient (case 7), the expansion of a 3.5-mm Multilink stent implanted in the right distal ICA was not adequate, and a 4.0-mm NC VIVA balloon with a length of 10 mm (Boston Scientific/SciMed) was used to further expand both the stent and the lesion. Procedural and clinical success rates were 83% (10/12) and 80% (8/10), respectively. No morbidity or mortality was encountered. The two failures (one in the IVA and one in the BA) occurred because neither the balloon nor the stent catheter could be navigated to the lesion owing to extremely tortuous proximal arterial segments. Consequently, 10 lesions in eight patients were eligible for arteriographic follow-up. All eight patients in whom stenting was successful underwent arteriographic follow-up 3 months after the procedure (100% follow-up). The diameter stenosis rate at that time was 19 ± 9%, which increased by 12% relative to the immediate post-CAS angiogram (P < .001) (Fig 4). No arteriographic restenosis occurred at 3 months. No transient ischemic attacks or strokes occurred during the 11 months' (average) follow-up period.
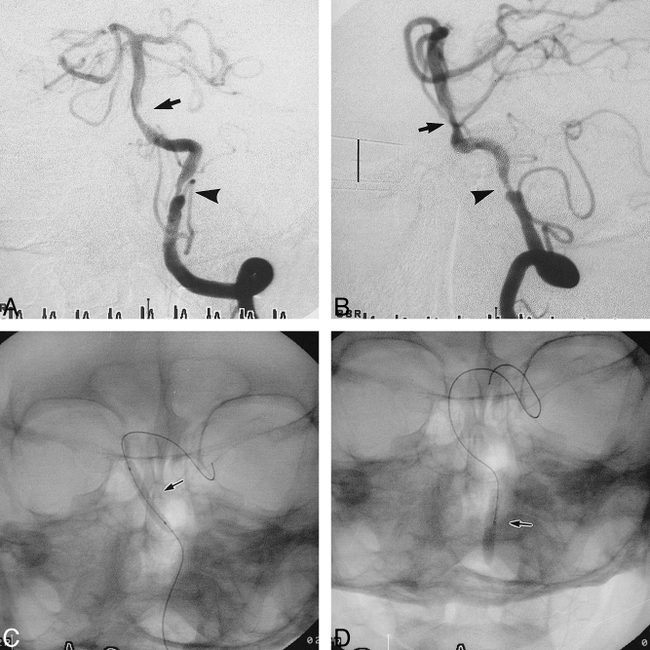
Case 6.
A and B, Anteroposterior (A) and lateral (B) views of the left vertebral arteriogram before stent placement reveal a tubular (9.3 mm in length) and moderately eccentric stenosis (62%) (arrow) of the BA and a tubular (8.3 mm in length) and moderately eccentric stenosis (77%) (arrowhead) of the left IVA. Scale (B): 10 mm.
C and D, Anteroposterior radiographs show the 3.0-mm gfx stent during deployment in the BA (C) and the 3.5-mm Ranger balloon further expanding the gfx stent in the left IVA (D).
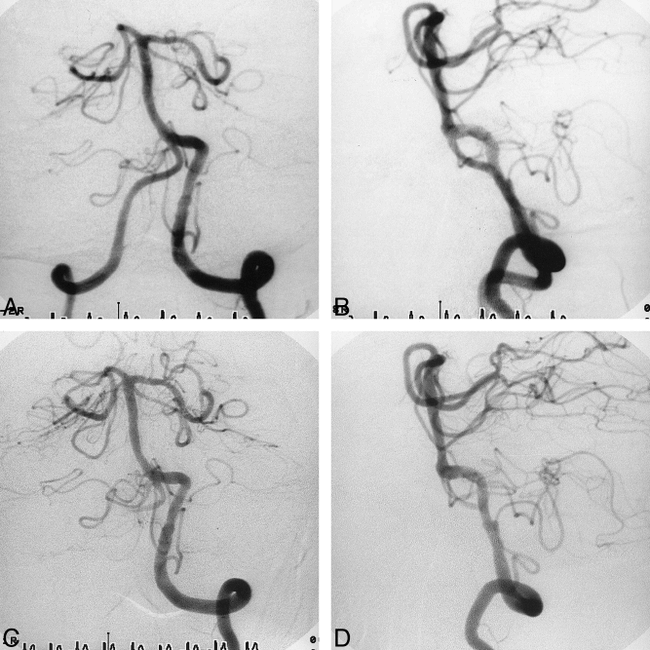
Case 6.
A and B, Anteroposterior (A) and lateral (B) views of the left vertebral arteriogram immediately after CAS show sufficient and smooth dilatation of both lesions.
C and D, Anteroposterior (C) and lateral (D) views of the left vertebral arteriogram 3 months after CAS show no restenosis of either lesion.
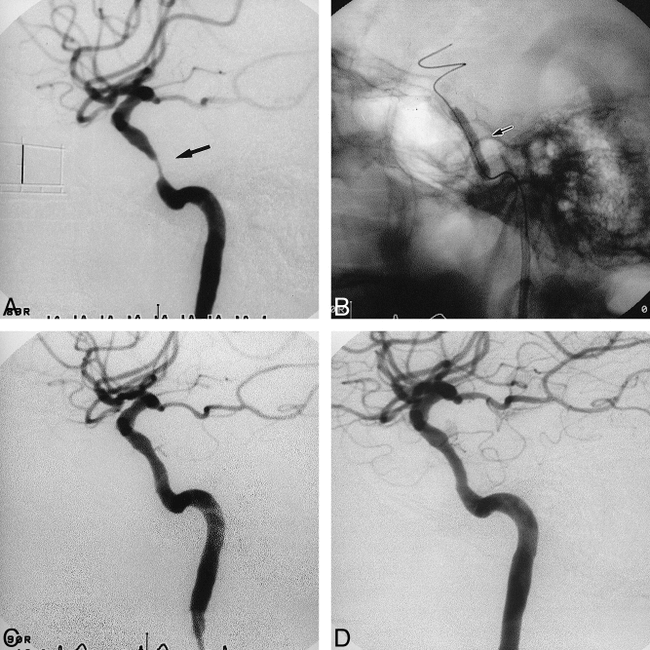
Case 7.
A, Lateral view of the right carotid arteriogram before stent placement reveals a tubular (11.4 mm in length) and eccentric stenosis (82%) (arrow) of the distal ICA. Scale: 10 mm.
B, Lateral radiograph shows a 4.0-mm NC VIVA balloon catheter further expanding the 3.5-mm Multilink stent in the right distal ICA (arrow).
C, Lateral view of the right carotid arteriogram immediately after stent placement shows sufficient and smooth dilatation of the lesion.
D, Lateral view of the right carotid arteriogram 3 months after stent placement shows no restenosis.
Changes in the percentage of diameter stenosis on the angiograms obtained before (pre-CAS) and immediately after (post-CAS) treatment and at the 3-month follow-up. There is significant reduction of the stenosis rate between the pre-CAS and immediate post-CAS angiograms (P < .0001, paired Student's t-test)
Discussion
Our results suggest that CAS is a safe and effective means for treating intracranial atherosclerotic lesions of the vertebrobasilar artery and the distal ICA, and that short-term arteriographic or clinical outcome after CAS is favorable.
Anticipation of the likelihood of a successful procedure is required before performing elective PTCBA. A cerebral arteriographic classification (12, 13) has categorized lesions by their length and geometry as follows: type A, short (5 mm or less in length), concentric or moderately eccentric, and less than totally occlusive; type B, tubular (5–10 mm in length), extremely eccentric, and moderately angulated (curved), or chronic and totally occlusive for less than 3 months; and type C, diffuse (>10 mm in length), extremely angulated (>90°) with an excessively tortuous proximal segment, or chronic and totally occlusive for 3 months or longer. Before CAS, 12 lesions in the present study were categorized according to those arteriographic characteristics: eight were classified as type B, two as type C, and two produced acute total occlusion (see Table).
A previous study (13) reported that the clinical success rates of standard elective PTCBA for type B and C lesions were 86% and 33%, respectively. Arteriographic restenosis rates 1 year after elective PTCBA for type B and C lesions were 33% and 100%, respectively. Cumulative risks of fatal or nonfatal ipsilateral ischemic stroke 1 year after elective PTCBA for type B and C lesions were 12% and 56%, respectively. For lesions in the type B and C groups, procedural complications and restenosis limited in-hospital and long-term arteriographic and clinical outcomes. To avoid abrupt closure or symptomatic dissection and to retain long-term patency, we made an attempt to apply a coronary stent in the type B and C lesions and in the cases of acute total occlusion that seemed to be unresponsive to standard PTCBA. The gfx and Multilink coronary stents, which have good flexibility and are of an appropriate length to follow arterial tortuosity, can be made to conform to vascular morphology. In addition, their struts, with wide openings, do not seem to occlude side branches, such as perforating arteries. The Ranger coronary balloon catheter, with its good flexibility, tractability, and over-the-wire system, was used to predilate the intracranial lesions. The clinical success rate of CAS was 83% (10/12), and the arteriographic restenosis rate was 0% (0/10) at 3 months. Cumulative risk of fatal or nonfatal ipsilateral ischemic stroke after CAS was 0% at 6 months. As compared with standard elective PTCBA for type B or C lesions, CAS with flexible coronary stents seems to yield favorable in-hospital and short-term arteriographic and clinical outcomes.
Reports on the use of CAS for an intracranial atherosclerotic or thrombosed vertebrobasilar artery are few (14–16), although some authors have described the application and efficacy of a balloon-expandable coronary stent for the treatment of distal carotid atherosclerotic stenoses (17–19). As yet, no stent has been specifically designed to treat intracranial atherosclerotic stenosis. If thinner and more flexible neurovascular balloon catheters and stents specifically designed for an intracranial lesion had been available, two patients in whom access failure occurred could have been treated successfully.
The present study has several limitations. There is no standardized method for measuring the extent of intracranial lesions, although cerebral arteriographic classification of lesion morphology by length and geometry is important to anticipate the success of the procedure. The number of patients was very small. Procedural morbidity of CAS depends on the technical skill of the person performing the intracranial angioplasty, and the technical skill in deploying stents improves with experience. Our study had no valid comparison group of patients who underwent standard PTCBA alone for similar lesions, making it hard to formulate strong inferences about the effect of CAS treatment. Additionally, distal propagation of emboli will still be a potential danger of CAS for treatment of atherosclerotic occlusive disease. Perforator occlusion might occur by forcing portions of debris into branch arteries if a balloon or stent is dilated in the atherosclerotic lesion. Intracerebral or intraventricular hemorrhage after balloon angioplasty or stenting for a carotid bifurcation stenosis has also been reported (20, 21). Long-term arteriographic and clinical outcome after CAS treatment is uncertain. Therefore, stent placement in the intracranial atherosclerotic artery must be applied carefully.
Conclusion
CAS with flexible balloon-expandable stents may prove to be a safe and effective means for treating intracranial atherosclerotic lesions of the vertebrobasilar artery and the distal ICA, and may yield a favorable short-term arteriographic and clinical outcome. Our results warrant further investigation of CAS and stent technology.
Acknowledgments
We thank Yasufumi Uchida, Akihito Moriki, Hirohisa Miyake, and Hiroyuki Nishimura, Department of Neurosurgery, Uchida Neurosurgical Hospital; Masayuki Kuwahara, Department of Neurosurgery, Kohoku Hospital; Kazuumi Tamura, Department of Neurosurgery, Tosa City Hospital; Masahiro Kurisaka, Masanori Morimoto, and Masato Seike, Department of Neurosurgery, Kochi Medical School; and Masaaki Fukuoka, Department of Neurosurgery, Nishimura Neurosurgical Hospital, for supporting our work. We also thank Noboru Yamamoto, Kenzi Ito, Masanori Takemura, and other staff members in the catheter laboratory, Kochi Medical School Hospital, for supporting us.
Footnotes
↵1 Address reprint requests to Takahisa Mori, MD, PhD, Division of Neurological Endovascular Intervention, Department of Neurosurgery, Kochi Medical School Hospital, Kohasu, Okoh-cho, Nankoku City, Kochi 783-8505, Japan.
References
- Received June 25, 1999.
- Copyright © American Society of Neuroradiology