Abstract
BACKGROUND AND PURPOSE: Destructive lesions of the sinonasal tract, lacking a discernible etiology and referred to as midline destructive disease, have been pathologically classified in accordance with a variety of confusing terms. Development of new pathologic concepts and immunohistochemical techniques has provided a fresh understanding of these lesions, and, as a result, they can be unified into two distinct pathologic groups: Wegener's granulomatosis and non-Hodgkin's T-cell lymphoma.
METHODS: We retrospectively reviewed the imaging studies and pathologic specimens of seven patients with prior diagnoses included in the midline destructive disease group. The specimens were reviewed by an oral pathologist using currently accepted pathologic criteria and the newly available immunohistochemical markers CD20, CD45, and CD45RO. Lesions were classified as non-Hodgkin's T-cell lymphomas when positive for CD45 and CD45RO and negative for CD20, and as Wegener's granulomatosis in the presence of noncaseating multinucleated giant cell granulomas and necrotizing vasculitis.
RESULTS: Three of the lesions were reclassified as Wegener's granulomatosis and four as T-cell lymphomas after applying these pathologic criteria. There were no distinguishing imaging findings between Wegener's granulomatosis and non-Hodgkin's T-cell lymphoma.
CONCLUSION: The current pathologic classification for midline destructive disease should be incorporated into the radiologic lexicon and the use of terms from the old classification system, such as idiopathic midline granuloma and lethal midline granuloma, should be abandoned and no longer be used in radiologic reports.
The histopathologic classification of midfacial destructive lesions has undergone substantial revision within the last two decades. Advances in immunocytochemical phenotyping and molecular genetics have revealed that the majority of these lesions are in fact either a form of non-Hodgkin's lymphoma arising in the sinonasal tract or Wegener's granulomatosis (1–5). Terms used to refer to such lesions include Stewart's syndrome, lethal midline granuloma, idiopathic midline granuloma, idiopathic midline destructive disease, midline nonhealing granuloma, polymorphic reticulosis, lymphomatoid granulomatosis, and others, which previously represented destructive sinonasal lesions that could not be classified under specific pathologic entities based on histopathologic architecture alone (1–6). These older terms have been replaced in the pathologic diagnostic nomenclature of sinonasal disease by new terminology that accurately describes the cellular lineage and biological growth rate.
Unfortunately, this improved pathologic classification of midfacial destructive lesions has not been assimilated into the diagnostic nomenclature used by radiologists. Descriptions in current head and neck radiologic literature continue to rely on outdated terminology in reference to these lesions. Such terminology is not useful to clinicians in the management of patients with midfacial destructive lesions, and may even lead to costly delays in diagnosis and treatment.
To assess the validity and accuracy of the currently accepted pathologic classification and modern immunocytochemical phenotyping, we identified historical cases of midfacial destructive lesions that had previously been classified under the old terminology and re-examined the imaging studies and pathologic specimens with current histopathologic diagnostic techniques. By establishing that nearly all cases of idiopathic sinonasal destructive lesions are due to either lymphoma or Wegener's granulomatosis, we hope that the diagnostic terminology now used by histopathologists will also become the standard for radiologic diagnosis and classification of such lesions.
Methods
Seven cases of midfacial destructive lesions were identified through a computerized search of the Pathology Reporting Office database at the UCLA Center for Heath Sciences. The period searched ranged from January 1955 through September 1989. The terms used in this search included Stewart's syndrome, midline granuloma syndrome, malignant midline granuloma, lethal midline granuloma, idiopathic midline granuloma, idiopathic midline destructive disease, nonhealing midline granuloma, polymorphic reticulosis, lymphomatoid granulomatosis, and pseudolymphoma. The initial pathologic diagnosis was made by tissue sampling from the sinonasal tract in all patients included in this series.
The histologic slides of each specimen were prepared for immunohistological stains. All the slides were reviewed by the same oral pathologist and stained with B- and T-cell markers, including CD20, CD45, and CD45RO. Antineutrophil cytoplasmic antibody (ANCA), a serologic marker for Wegener's granulomatosis, was not available for this population. In some selected cases, additional stains were performed to exclude other possible pathologic entities. These stains included synaptophysin and chromogranin to exclude other small cell tumors (neuroendocrine carcinoma and esthesioneuroblastoma), HMB45 to exclude melanoma, desmin and myoglobin to exclude alveolar rhabdomyosarcoma, and several cytokeratin stains to exclude small cell epithelial-derived carcinomas. In situ hybridization techniques for Epstein-Barr virus–related RNA were not performed. The original pathologic classification was then compared with the diagnosis based on new techniques. Medical records of those cases rediagnosed as T-cell lymphoma were reviewed to exclude the presence of systemic involvement at presentation.
The criteria used to establish the diagnosis of non-Hodgkin's T-cell lymphoma included the presence of a monoclonal lymphocytic infiltrate staining positively for CD45 and CD45RO and staining negatively for CD20. The pathologic diagnosis of Wegener's granulomatosis was made in the presence of multinucleated giant cell granulomas associated with a necrotizing vasculitis.
The original imaging studies of these patients, six CT scans and four MR images, were also reviewed by two radiologists who were blinded to the final diagnosis. Imaging features evaluated included the amount of soft-tissue thickening in the sinonasal tract, the amount of bone destruction, the ratio between the amount of soft-tissue and bone destruction, and the extent of radiologic involvement of the sinonasal tract.
Results
The initial and final pathologic diagnoses of the seven cases included in the study are presented in Table 1. After pathologic review and use of special immunohistochemical stains, all seven cases were reclassified into two different specific physiopathologic entities: non-Hodgkin's T-cell lymphoma (n = 4) and Wegener's granulomatosis (n = 3). All cases of T-cell lymphoma showed a monoclonal lymphocytic proliferation that stained positively for CD45 and CD45RO and stained negatively for CD20. Lymphocytic angioinvasion and bony invasion was demonstrated in one case (Fig 1B and C). None of the cases reclassified as T-cell lymphoma showed evidence of systemic involvement at presentation. All cases reclassified as Wegener's granulomatosis showed noncaseating multinucleated giant cell granulomas and necrotizing vasculitis associated with a mononucleate inflammatory infiltrate composed mainly of histiocytes and eosinophils. In all these cases, CD20, CD45, and CD45RO stainings were negative. In two cases, areas of fibrinoid necrosis were also present.
Diagnosis before and after review of the pathologic specimens and immunohistochemical analysis in seven patients with destructive lesions of the sinonasal tract
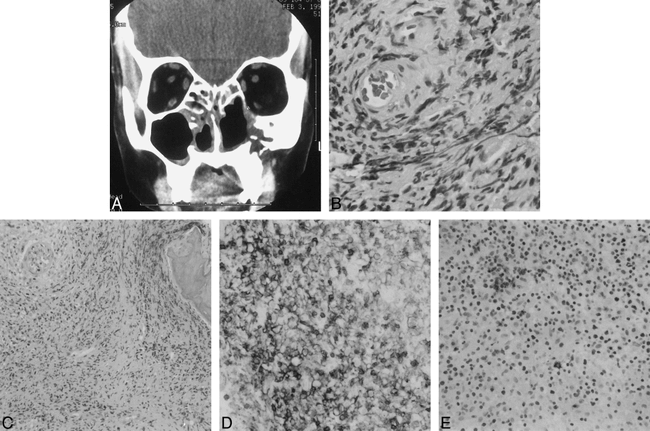
Patient 5. Initial diagnosis: nonhealing midline granuloma (Stewart's syndrome); final diagnosis: non-Hodgkin's T-cell lymphoma.
A, CT scan of the paranasal sinuses shows soft-tissue opacification of the anterior ethmoidal air cells bilaterally, soft-tissue density in the region of the right ostiomeatal unit, and mild mucosal thickening in the inferior aspect of the right maxillary sinus. The left maxillary sinus is hypoplastic and the remainder of the sinus cavity is filled with soft tissue. Bony destructive changes are noted with a nasoseptal perforation and destruction of the inferomedial wall of the right orbit. However, the abnormal soft tissue does not appear to infiltrate the extraconal space. The bony defect in the inferior aspect of the left maxillary sinus is postsurgical in nature, due to a prior Caldwell-Luc antrostomy for chronic sinus disease. Some soft-tissue thickening is noted in the left premaxillary region.
B, Histologic slide of a sinonasal biopsy specimen shows lymphocytic invasion of a blood vessel reflecting the angioinvasive nature of the disease. Also noted is a heterogeneous and polymorphic inflammatory infiltrate (hematoxylin-eosin, original magnification ×400).
C, Histologic slide of a sinonasal biopsy specimen shows invasion of trabecular bone in the right upper corner of the image (hematoxylin-eosin, original magnification ×100).
D, Histologic slide of a sinonasal biopsy specimen shows positive staining with CD45RO T-cell marker (original magnification ×200).
E, Histologic slide of a sinonasal biopsy specimen shows negative staining with CD20, a B-cell marker (original magnification ×200).
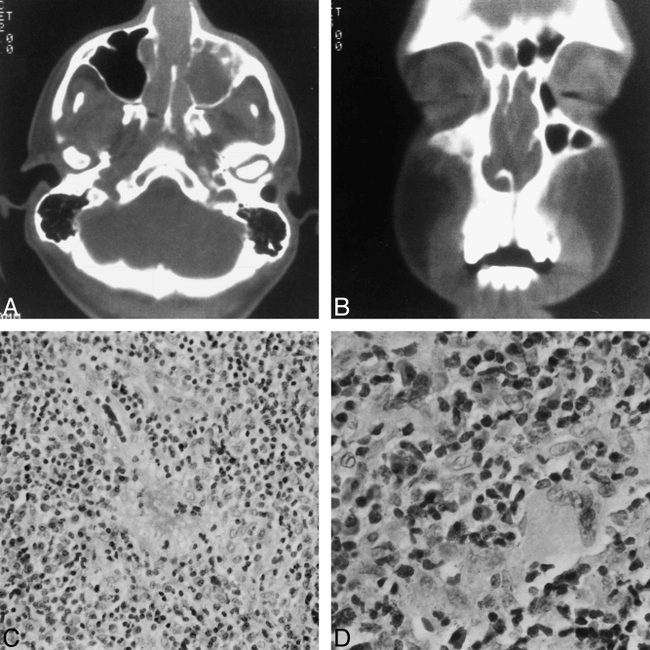
Patient 4. Initial diagnosis: malignant midline granuloma; final diagnosis: Wegener's granulomatosis.
A and B, Axial (A) and coronal (B) CT scans of the paranasal sinuses (bone window) show complete soft-tissue filling of the nasal cavity and nasal vault extending posteriorly to the choana and high nasopharynx. The anterior aspect of the bony nasal septum has been destroyed. The left maxillary sinus is totally opacified and slightly expanded with mild bowing of the medial wall of the sinus. The bony walls of the left maxillary sinus are intact. No abnormal soft tissue is seen in the retroantral fat or pterygopalatine fossa.
C, Histologic slide of a sinonasal biopsy specimen shows an inflammatory infiltrate composed mainly of histiocytes and eosinophils. Note also an area of fibrinoid necrosis in the center of the slide (hematoxylin-eosin, original magnification ×200).
D, Histologic slide of a sinonasal biopsy specimen shows typical multinucleated giant cells. A final diagnosis of Wegener's granulomatosis was based on the histologic findings and on necrotizing vasculitis (hematoxylin-eosin, original magnification ×400).
Review of the imaging studies of these cases did not show differences in the features evaluated. However, the group is too small to infer statistically significant conclusions from the radiologic findings. The results were qualitatively consistent with reports in the literature. All cases showed increased soft tissue within the sinonasal area. Three of the four patients with lymphoma and all the patients with Wegener's granulomatosis had septal perforation. Three of the four patients with lymphoma and one of the three patients with Wegener's granulomatosis had other nonseptal bone destruction involving the paranasal sinuses.
Discussion
History
The histopathologic classification of midline destructive lesions has undergone substantial revision over the last two decades. Historically, destructive midfacial lesions, other than those caused by trauma, toxic agents, or infectious or neoplastic processes, were categorized under multiple pathologic labels that did not reflect specific clinicopathologic entities and provided little guidance in disease management.
A confusing variety of terms has been used since 1897, when McBride first described a case of rapid destruction of the face and nose (7). This was followed in 1922, by Stewart's report of 10 cases of a chronic midfacial destructive process, which became known as Stewart's syndrome or Stewart's granuloma. In 1949, Williams popularized the unfortunate term lethal midline granuloma to designate inflammatory midline destructive lesions with no known etiologic factors. Soon after, it became clear that many of the disease processes included under this heading were neither lethal nor granulomatous. This awareness led to the propagation of other nonspecific descriptive terms, such as idiopathic midline granuloma, nonhealing midline granuloma, malignant midline granuloma, and idiopathic midline destructive disease, which were used indiscriminately and which referred to a relatively heterogeneous group of disease processes (Table 2).
Terms to be abolished from the radiologic lexicon
Advances in general medical knowledge, and in biochemical and pathologic analysis in particular, led to progressive separation of more specific disease entities from these general nonspecific headings. The first pathologic entity to be clearly distinguished was Wegener's granulomatosis, after Friedmann's histopathologic review of a variety of cases of this disease in 1955 (8, 9). The pathophysiological mechanisms involved in this disease and specific diagnostic criteria were also described (10, 11).
Later, another pathologist, Eichel (12, 13), differentiated another disease process from the idiopathic midline destructive disease group: polymorphic reticulosis, or lymphomatoid granulomatosis. In 1966, he defined this entity as a transitional pathologic process between atypical lymphoid proliferation and frank malignant lymphoma (12–14). The term pseudolymphoma has also been used to refer to this entity.
In the 1970s and 1980s, midline destructive diseases other than Wegener's granulomatosis were subclassified into three different groups that reflected variations in clinical behavior and histology (8). The first group, idiopathic midline destructive disease, was characterized as a locally destructive lesion limited to the upper respiratory tract and with no systemic involvement. The pathologic hallmark was the presence of nonspecific inflammation and necrosis with absence of granulomas and malignant cells (8). Polymorphic reticulosis, or lymphomatoid granulomatosis, the second group, was defined pathologically by the presence of an atypical and polymorphic lymphoid cell population associated with lymphomalike angiocentric and angioinfiltrative growth pattern, causing extensive necrotic changes (10). The last group, extranodal non-Hodgkin's lymphoma, included the cases of frank lymphoma. This was the classification still proposed by Batsakis in 1979 (3, 15).
During the 1980s, further advances in immunohistochemical phenotyping and in molecular genetics allowed better characterization of the cell surface and cell origin. In 1982, Ishi et al (16), using immunofluorescent studies and a variety of antisera directed toward human T- and B-cell surface antigens, definitely linked polymorphic reticulosis with non-Hodgkin's lymphoma. In 1987, Lippman demonstrated activated T-cell phenotype with the typical pattern of peripheral T-cell lymphoma in a case of midline destructive granuloma (17).
In the 1990s, in situ hybridization techniques, gene rearrangement studies, and the discovery of cellular expression of specific cellular membrane molecules allowed for further confirmation of the nature of these lesions (4, 18, 19).
Diagnosis
The diagnosis of destructive diseases of the sinonasal region depends on clinical and pathologic findings, as imaging of these lesions is nonspecific (11, 20–22). Patients usually present with symptoms of nasal obstruction and nasal discharge, which are easily attributed to rhinosinusitis. Epistaxis and facial swelling may be in the clinical spectrum. With disease progression, facial pain and destructive sinonasal lesions may ensue. On physical examination, the most typical finding is the presence of nasal septum perforation, which may or may not be associated with soft-tissue masses and which may progress to autorhinectomy (3, 5, 20).
After assessing the extent of disease with sectional imaging, the first step should be to exclude more common etiologies, such as trauma (accidental or self-induced), cocaine abuse, and infection (Table 3). These may be excluded through clinical history and culture of sinonasal secretions. General systemic symptoms may be present in both sinonasal lymphoma and Wegener's. They include fatigue, night sweats, and weight loss. Although lung and kidney involvement are common in Wegener's granulomatosis, sinonasal disease may be the presenting feature and may antedate involvement of other organs (10, 11). The specific marker of Wegener's granulomatosis, antineutrophil antibodies, may be absent in the serum and confound the diagnosis (11). In this circumstance (negative ANCA and isolated involvement of the sinonasal tract), the diagnosis of Wegener's is based on histologic features, including the presence of noncaseating multinucleated giant cell granulomas and necrotizing vasculitis. Fibrinoid necrosis may or may not be present.
Differential diagnosis of destructive lesions of the sinonasal tract
Sinonasal lymphoma is one of the rarest forms of extranodal lymphoma in Western populations, representing less than 0.5% of cases (3). This contrasts with the prevalence in some Asian countries, in which sinonasal lymphoma is the second most common type of extranodal lymphoma. In this geographic group, over 90% of cases have T-cell markers, and Epstein-Barr virus has been consistently demonstrated in the cell genome (3).
The diagnosis of sinonasal non-Hodgkin's T-cell lymphoma is based on the presence of a monoclonal lymphocytic proliferation and specific immunohistochemical markers (CD45 and CD45RO). Angio- and bony invasion is a typical feature of T-cell lymphomas in this region, thus accounting for the term angiocentric lymphoma (2, 3).
Recently, Barker et al (23), reported a single case of idiopathic destructive sinonasal disease that did not conform to the diagnostic criteria of either Wegener's granulomatosis or T-cell lymphoma. It is not clear from this case report what the specific characteristics were of the centrally necrotic granuloma found in one of the nasal biopsy specimens. The authors attributed it to a reaction to previous surgery or topical medication. However, as discussed above, granulomas may be part of the definite diagnostic criteria of early or atypical Wegener's (with negative ANCA and without involvement of other organs). Also, the control of the disease with cyclophosphamide and prednisolone, the recommended therapy for Wegener's granulomatosis, may argue in favor of this disease. Whether cases such as this one represent diagnostic insufficiency or a separate pathologic entity remains elusive and stands in the face of a growing body of literature to suggest otherwise (3, 4, 18, 19).
In the absence of a definite diagnosis, biopsies should be performed and special stainings used as needed. Both T-cell lymphoma and Wegener's granulomatosis may exhibit foci of necrosis and dense mononuclear infiltrates. Specific immunohistochemical markers are useful in establishing a definite diagnosis, particularly when secondary inflammatory changes are extensive. One important concern in tissue sampling is to perform deep biopsies in order to avoid the necrotic and inflammatory components of the lesion. These two factors most likely contributed to the nonspecificity of pathologic findings in the past. Biopsies performed under imaging guidance may be required to improve the diagnostic yield.
Imaging
Imaging of early midline destructive lesions (Figs 1A and 2A and B) is likely to reveal little more than nonspecific findings of mucosal thickening, suggestive of chronic sinonasal inflammation. Bony erosion and destruction are the hallmarks of aggressive lesions but are still nonspecific, eliciting a long differential diagnosis (Table 2). Such destruction is typically seen to first involve the nasal septum and occasionally to spread to the paranasal sinuses, more commonly to the medial wall of the maxillary sinus and ethmoidal trabecula. Advanced disease may lead to destructive lesions of the hard palate, sinonasal-oral fistulas, or complete nasal destruction (autorhinectomy), which is more specific for angioinvasive nasal lesions, such as lymphoma and Wegener's granulomatosis, but can also be seen in angioinvasive fungal infections, such as aspergillosis and mucormycosis. After transgressing bony landmarks, these pathologic processes may extend to adjacent structures. The premaxillary soft tissues, retroantral fat, pterygopalatine fossa, infratemporal fossa, and orbit are the most commonly involved. Intracranial involvement may also result, usually involving the anterior cranial fossa, owing to spread of disease through the cribriform plate.
It is apparent from the radiologic literature that several attempts have been made to distinguish the various types of midline destructive processes on the basis of imaging findings. In 1989, Drake-Lee and Milford (21), after reviewing the plain films and CT scans of 20 cases of Wegener's granulomatosis and seven cases of lethal midline granuloma, concluded that these diseases have no specific radiologic features and that the differences between the two pathologic process were quantitative, with more extensive destructive changes seen in midline destructive disease than in Wegener's.
In 1990, Teng et al (14) described the CT findings in a series of 11 cases of polymorphic reticulosis and concluded that the imaging features were nonspecific. In 1991, Marsot-Dupuch et al (22) described and tried to quantify imaging findings in 13 cases of lethal midline granuloma evaluated with CT and MR imaging. These authors concluded that there are no specific imaging findings and that the main role of imaging this disease is to evaluate the extent of the disease, monitor its progression over time, and ascertain the effect of treatment. They also concluded that CT with high-resolution bone algorithms is the best method to evaluate bony changes, such as remodeling and erosion, and that MR imaging should be used to determine the extent of soft tissue, orbital, and intracranial involvement. They also concluded that MR imaging is useful for distinguishing fluid retention within the sinuses from mucosal thickening and intranasal masses.
The present series was too small to attempt to compare and quantitate the imaging findings; these are highly dependent on the progression of the disease before treatment. In both Wegener's granulomatosis and sinonasal lymphoma, extensive destructive changes, including autorhinectomy, were seen.
Conclusion
Advances in immunocytochemical phenotyping have enabled pathologists to greatly simplify the terminology for midline destructive lesions of the sinonasal tract. Because of immunohistochemical and gene rearrangement analysis, we now know that the vast majority of patients suffering from non-Wegener's idiopathic midline destructive lesions have lymphoma of the sinonasal tract. Thus, radiologists should refrain from using pathologic terminology that is no longer valid and instead provide an accurate description of the disease process and a list of differential diagnoses that, once other pathologic entities easier to diagnose have been excluded, includes sinonasal lymphoma and Wegener's granulomatosis, alerting the clinician to these possibilities and encouraging the use of immunohistochemical and serologic markers specific for these diseases.
Footnotes
↵1 Address reprint requests to Alexandra Borges, MD, Department of Radiology, PU54, Head & Neck Room, B3227P, UCLA School of Medicine, Los Angeles, CA 90024.
References
- Received August 21, 1998.
- Accepted after revision September 13, 1999.
- Copyright © American Society of Neuroradiology