Abstract
Summary: Aneurysm models were used to study the density of packing after coil embolization. Platinum coils were introduced until the point of minimally dense packing, indicated by aneurysmal circulatory exclusion. Packing was continued up to the point of maximal density, indicated by protrusion into the parent artery. Volumetric ratios (coil volume/aneurysmal volume) were calculated for minimally and maximally dense packing. Maximally dense packing ratios were a little higher than the minimally dense ratios, but less than 37%.
Detachable coils are now widely used for the treatment of cerebral aneurysms. When the aneurysm is not tightly occluded, however, the coils have a propensity to gather together, being pushed and displaced toward the dome by the arterial pulsatile flow. To avoid this problem, dense packing has been proposed. There is no definition of “dense packing” and nobody knows exactly to what extent coils can be placed into the aneurysmal cavity. The aim of this experiment was to perform precise in vitro volumetric measurements to define dense packing of small aneurysms with detachable platinum coils.
Technique
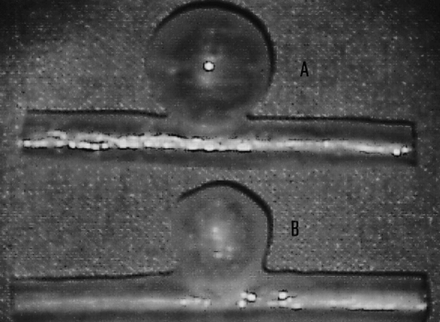
Two different kinds of in vitro silicone sidewall aneurysm models were made using the method of Gailloud et al (1). The aneurysm models had a parent vessel with an internal lumen of 5 mm and a lateral spherical aneurysmal cavity with an internal diameter of 10 mm for the small type (neck size, 3 mm) and 12 mm for the larger one (neck size, 5 mm) (Fig 1). Four small aneurysms (models 1, 2, 3, and 4) and four large ones (models 5, 6, 7, and 8) were made using the same technique. To measure the volume of each aneurysm precisely, a special micropump capable of injecting very small amounts of liquid with an accuracy of 1.0 ng/mL was developed. Measurements of aneurysmal volume were carried out with the parent artery horizontal and the dome of the aneurysm vertical, so that the plane of the orifice of the aneurysm was horizontal. A Tracker-18 microcatheter (Target Therapeutics, Fremont, CA) was placed into the model so that its tip was just at the level of the orifice of the aneurysm. Then stepwise filling of the sac was achieved with contrast medium under fluoroscopic control (Philips Integris V 3000 BN, Best, the Netherlands), until the surface of the fluid was just at the level of the orifice. This infusion was repeated five times per model, and we regarded the average volume as the definitive aneurysmal volume.
Fig 1. Soft silicone models of sidewall aneurysms (A, 12-mm-diameter model; B, 10-mm-diameter model)
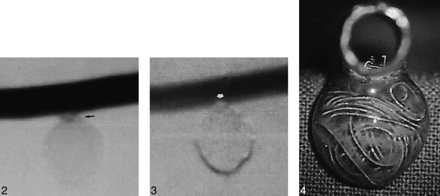
The aneurysm model was then connected to a circulatory system and to a pump (Drapier, Collin, France), which provided a pulsatile flow. Pressure values delivered by the pump were set to match physiological conditions. The circulating fluid (normal saline solution) was kept at 37°C. Under fluoroscopic guidance, the Tracker-18 microcatheter was navigated to the aneurysm's orifice. Dense packing of the aneurysm was then attempted with the mechanically detachable spiral platinum coils (DCS-18, William Cook Europe A/S, Bjaeverskov, Denmark). Digital subtraction angiography was performed after each coil was introduced. Minimally dense packing was considered to occur when circulation within the aneurysm appeared to have ceased angiographically (Fig 2). Maximally dense packing was defined as the point at which introduction of an additional coil caused slight protrusion into the parent artery (ie, the point at which more coils could not be safely placed using the standard technique of coil embolization) (Figs 3 and 4). Dense packing was achieved in four aneurysms, two small and two large. Calculation of the total volume of the coils introduced into each aneurysm was based on a 0.015-inch diameter, corresponding to 1.140 mm3/cm of coil. Then the volumetric ratios of minimally and maximally dense packing with respect to the actual aneurysmal volume were calculated.
Fig 2. Digital subtraction angiogram of model 2 after minimally dense packing (total coil length, 134 cm; ratio, 32.51%). Some diluted contrast medium is seen at the neck, attesting to the suboptimal filling of the sac (arrow).Fig 3. Digital subtraction angiogram of model 2 after maximally dense packing (total coil length, 149 cm; ratio, 36.15%). The orifice of the aneurysm is occluded (arrow).Fig 4. Tangential view of model 2 after maximally dense packing. Slight coil protrusion is seen within the parent vessel lumen
The raw data used to calculate the volumes of the aneurysm models are shown in Tables 1 and 2. The average aneurysmal volumes, coil lengths and volumes, and volumetric ratios from the embolization experiments are shown in Tables 3 and 4. Volumetric ratios for minimally dense packing were approximately 26% to 33% and those of maximally dense packing were about 30% to 36%.
Results of volume (mm3) determination of the four 10-mm-diameter aneurysm models
Results of volume (mm3) determination of the four 12-mm-diameter aneurysm models
Results of minimally dense packing
Results of maximally dense packing
Discussion
The goal of endovascular treatment of cerebral aneurysms is to obtain a complete, stable exclusion of the sac from the arterial circulation, with preservation of the parent vessel. Ideally, aneurysmal thrombosis followed by endothelialization across the aneurysmal orifice should be obtained. Coils are now used extensively to treat ruptured aneurysms in the acute stage. Viñuela at al (2), in a series of 403 patients with ruptured aneurysms, reported a risk of rebleeding of 2.2% within 6 to 36 months in patients in whom aneurysms were incompletely occluded with Guglielmi detachable coils (GDCs). There are, unfortunately, very few clinical series in which early and late histopathologic findings after endovascular therapy with coils have been reported (3–5). Animal studies have yielded contradictory results concerning thrombus formation, presence or lack of persistent thrombus, and healing of the arterial wall with endothelialization at the site of the neck (6–8). Incompletely coiled aneurysms, therefore, can potentially rebleed after treatment (9). Coil thrombogenicity has been considered a favorable characteristic for aneurysmal exclusion (10). Although an aneurysm packed with coils may appear radiographically dense, experimental studies have indicated that a significant part of the sac becomes acutely occluded with thrombus after coiling. This clot has no permanency in some cases, exposing the sac to the possibility of recanalization and coil compaction. Graves et al (11) reported this phenomenon in dogs. Coil packing is an important factor on which the long-term stability of aneurysmal occlusion after endovascular treatment relies (2), although animal models have shown a propensity for recanalization in less densely packed aneurysms and a positive relationship between packing density and long-term occlusion rate.
The Concept of Minimally Dense Packing
We defined minimally dense packing as the point at which internal aneurysmal flow stopped. At this point, no further filling of the aneurysmal cavity with contrast material could be seen angiographically. Partial coil embolization induces major flow disturbances in the aneurysm; in particular, fluid stagnation at the dome. Partial coil embolization may prevent early rebleeding; however, we think it is insufficient to prevent long-term protection against rehemorrhage.
Volume of Coils with Respect to Aneurysmal Volume
After embolization, visual inspection of the models revealed gaps within the coil mesh, after both minimally and maximally dense packing. Coils have a circular memory and do not necessarily enter residual spaces in the sac during coiling. This explains why little difference was seen between volumetric ratios of minimally and maximally dense packing, and why the ratios were so low, even after reaching the point of maximally dense packing.
Limitations of the Experiment
Although most aneurysms are located at arterial bifurcations, and our models were of sidewall aneurysms, we think they were satisfactory for the basic purpose of the experiment; however, they did not reflect the conditions that are present in clinical situations. For instance, the circulating saline fluid was not as viscous as blood or clot in the same way. In vivo, the endosaccular thrombosis occurs with detachable coils, meaning that fewer coils are required to achieve minimally dense packing. Volumetric ratios of in vivo aneurysmal packing would therefore be expected to be lower. Also, several attempts to position the last coils were made in some cases to achieve maximally dense packing. More tentative packing is usually employed in clinical settings to avoid aneurysmal perforation, especially in cases of ruptured aneurysms. So the volumetric ratios with maximally dense packing in this study were perhaps not entirely realistic.
In vitro aneurysmal volumes can also be assessed accurately with different imaging techniques, including CT and rotational angiography (12). Conversely, it is impossible to measure in vivo the precise volume of aneurysms, partially because human aneurysms are irregular rather than spherical in shape. The size (0.015-inch: diameter of the spiral, 3–10 mm; length, 6–20 cm) and type (mechanically detachable) of the platinum coils do not correspond to those currently used in many centers (GDC-10: 0.010-inch standard, 0.0085-inch soft). The use of smaller (2-mm-diameter) and softer coils would certainly have resulted in better filling of the aneurysmal sac, subsequently increasing the volumetric ratios.
Technical Characteristics of the DCS Coil System Used
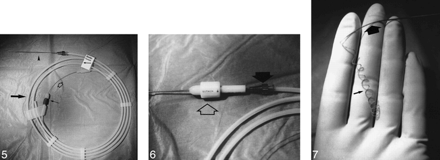
The DCS-18 coil system consists of an introducer with a premounted detachable platinum coil. The introducer system contains a stainless steel delivery wire, a flushable Detach Locking Device (DLD), and a delivery wire inserter, which consists of a plastic delivery wire holder with a delivery wire and a cannula inserter containing the coil (Figs 5-7,6). A safety-lock fitting prevents undesired movements of the system during transportation. At the distal end of the delivery wire, a thread is connected to the platinum coil. On the delivery wire, a 6-mm-long platinum marker is placed 3.0 cm behind the detachment zone between the coil and the delivery wire. The DCS is introduced through a microcatheter with a distal double marker. During coil positioning, this platinum marker is placed just behind the proximal marker of the delivery microcatheter, the junction between the coil and delivery wire is located exactly at the tip of the microcatheter to ensure safe detachment of the coil. Counterclockwise rotation of the DLD detaches the coil by unscrewing the thread from the coil.
Fig 5. Overview of the DCS in its package. Large arrow indicates delivery wire holder; open arrow, delivery wire; small arrow, safety-lock fitting; arrowhead, delivery wire inserter.Fig 6. Delivery wire inserter cannula (closed arrow) positioned into the DLD (open arrow). By turning the DLD counterclockwise approximately 25 turns, the coil detaches from the delivery wire.FIG 7. Delivery wire (large arrow) and spiral platinum coil (small arrow)
Conclusion
Even though our experimental conditions did not exactly reproduce the clinical setting, our results confirm the impression that much space is left within the aneurysmal sac after coiling a sidewall aneurysm as completely as possible. There is very little difference between the volume of coils required for minimally and maximally dense packing.
Acknowledgments
We are grateful to Alain Jacottet (Ecole Polytechnique Fédérale de Lausanne, Switzerland), who was instrumental in the conception and construction of the micropump system. The platinum coils were provided by Helene Quie (William Cook Europe A/S). We thank Ian B. Ross for reviewing the English.
Footnotes
References
- Received April 5, 1999.
- Copyright © American Society of Neuroradiology