Abstract
BACKGROUND AND PURPOSE: Aneurysm embolization using Guglielmi detachable coils (GDC) is gaining increasing acceptance as a viable alternative to surgery in the treatment of cerebral aneurysms. Although recent reports describe a significant rate of symptomatic thromboembolic complications with GDC use, many of the neurologic deficits are transient. We sought to determine the incidence of silent thromboembolic events with the use of diffusion-weighted imaging and to correlate radiologic findings with the results of neurologic examinations.
METHODS: Diffusion-weighted MR imaging was performed within 48 hours in 14 consecutive elective GDC aneurysm treatments. Embolizations were performed under systemic heparinization; all flush solutions were heparinized, and both guiding catheters and microcatheters were placed for continuous heparinized infusions. Neurologic examination, including the National Institutes of Health Stroke Scale determination, was performed by a stroke neurologist before the coiling procedures were performed, immediately after the procedures were performed, and before discharge. MR imaging examinations were reviewed by a stroke neurologist and an interventional neuroradiologist, with determination and characterization of diffusion-weighted imaging abnormalities.
RESULTS: Small areas of restricted diffusion, presumed to represent procedure-related embolic infarctions, were noted on the images of eight of 14 patients. All except one of the areas were located ipsilateral to the side of the catheterization. Six patients had evidence of multiple infarcts. Most lesions were small (<2 mm); one patient with coil stretch and herniation into the parent vessel had numerous infarcts with a dominant posterior frontal infarct. Pre- and posttreatment National Institutes of Health Stroke Scale scores were unchanged for 13 of 14 patients. Overall, the rate of asymptomatic emboli was 61% (eight of 13 treatments) in uncomplicated treatments. Strokes occurred independently of the number of coils used; the mean number of coils used for patients with strokes was 7.6 (range, two to 13) and for patients without evidence of infarcts was 10.2 (range, one to 30). This was not a significant difference (P > .5).
CONCLUSION: Silent thromboembolic events related to the use of the GDC system are a common occurrence, despite meticulous technique and systemic anticoagulation. Although clinical sequelae are rare, the high rate of occurrence suggests that alterations in the technique, such as the addition of antiplatelet agents, should be considered.
Advances in material sciences and medical imaging have allowed endovascular approaches to cerebral aneurysms to emerge as safe and effective alternatives to surgical clipping. For saccular aneurysms, the electrolytic Guglielmi detachable coil (GDC) system has been in clinical use for several years. The GDC allows for controlled placement of platinum coils into the lumen of a saccular aneurysm with subsequent local thrombus formation and obliteration of the aneurysmal sac (1). Previous reports (2–4) have emphasized the safety and efficacy of the GDC technique for the endovascular treatment of ruptured aneurysms. One potential source of complications with the technique is thromboembolic events, with both the catheters and coil mass acting as a possible thromboembolic source. Some more recent studies have assessed the technique's safety with both incidental and symptomatic cerebral aneurysms (5, 6). The results have been somewhat disparate, with thromboembolic complications ranging from 4.3% to 28%. Furthermore, microemboli, often without clinical accompaniment, have been detected by transcranial Doppler in 31% of patients during aneurysm coiling (7). These differences in detection rate may be dependent on inherent differences in the specificity and sensitivity of the techniques used for event detection. With its high accuracy for diagnosis of brain ischemia, diffusion-weighted MR imaging is ideally suited to identify and quantify thromboembolic events. The aim of the present study was to observe the incidence, size, location, and clinical sequelae of embolic injury, occurring perioperatively with aneurysm coiling, using diffusion-weighted MR imaging correlated with clinical examinations.
Methods
Patients
We prospectively evaluated all patients referred for endovascular coiling of cerebral aneurysms at our institution between November 1998 and October 1999. All patients with unruptured aneurysms suitable for endosaccular coiling that did not involve planned parent artery occlusion or stent placement were enrolled in the study (Table).
Clinical and radiologic characteristics
Continued
Each patient underwent a complete and detailed neurologic examination, including the National Institutes of Health Stroke Scale determination (8), performed by a stroke neurologist before and immediately after the embolization procedure and before discharge. In addition, patients were closely monitored in the neuroscience intensive care unit for any clinical changes during the first 48 hours after the procedure was performed.
GDC Technique
All GDC embolization procedures were performed with the patient under general anesthesia. Continuous arterial pressure measurements were obtained using a radial arterial line. The systemic blood pressure was regulated by the anesthesia team to maintain systolic blood pressure of ≤100 mm Hg. Cerebral angiography was performed by using a standard transfemoral approach to complete the evaluation of the cerebral arteries if this had not been done in a previous study. A single 6F femoral sheath was used, except when balloon remodeling was necessary, in which case bilateral 6F femoral sheaths were placed. After the placement of femoral sheaths, all patients were systemically anticoagulated with IV administered doses of heparin (typically 5000 U) to achieve prolongation of the activated clotting time (ACT) to >2.5 times baseline. Obtaining the ACT was repeated at least each hour through the procedure, and repeat boluses of heparin were administered as necessary to maintain the ACT at the targeted level. The sheath(s) and guiding catheter were continuously flushed with an infusion of heparin and saline solution (4000 U/L). After placement of a 6F guiding catheter into the cervical internal carotid artery or vertebral artery, control angiography was performed to determine a working projection for coil deployment. If balloon remodeling was anticipated (five patients required the use of this technique), the 6F guiding catheter was positioned in the common carotid artery, contralateral vertebral artery, or ipsilateral subclavian artery and a second 5F guiding catheter was positioned in the internal carotid artery or vertebral artery. The microcatheter was introduced through the guiding catheter and navigated into the aneurysm by using a fluoroscopic road map. No intraaneurysmal injections were administered. The microcatheter was also placed to a continuous heparinized drip. GDCs were placed under fluoroscopic guidance using standard techniques. After placement of each coil, angiography was performed to assess the degree of aneurysm obliteration, to assess the patency of the parent artery, and to look for signs of thromboembolic phenomena. Once the aneurysm packing was deemed complete, whole-head angiography was performed to assess for thromboembolic complications. Patients were kept heparinized with partial thromboplastin times of 60 to 80 seconds for 2 days postoperatively. For some patients, after 24 hours, the administration of heparin was temporarily discontinued for 2 to 3 hours for femoral sheath removal. The administration of aspirin (325 mg/day) was begun on the 3rd postoperative day. Patients were monitored in the intensive care unit after undergoing the procedure and generally were transferred to the floor on the 2nd postoperative day.
Diffusion-weighted MR Imaging
MR imaging was performed within 48 hours of the coiling procedure. All patients underwent our acute stroke imaging protocol, which includes sagittal T1-weighted, axial T2-weighted, proton density–weighted, or fluid-attenuated inversion-recovery imaging plus diffusion-weighted imaging. Our diffusion-weighted imaging technique samples the entire diffusion tensor (9). The technique consists of obtaining six high-b-value single-shot images at each section position, each corresponding to a diffusion measurement in a particular direction, and then a single low-b-value image. The high b value is 1221 s/mm2; the low b value is 3 s/mm2. The parameters were as follows: 6000/118 [TR/TE]; matrix, 256 × 128; field of view, 40 × 20 cm; section thickness, 6 mm; intersection gap, 1 mm. The complete seven-image tensor acquisition requires 42 seconds; we typically acquire three repetitions to improve the signal-to-noise ratio, which results in a total imaging time of 126 seconds. Generation of isotropic (tensor trace) diffusion-weighted images occurs off-line on a networked workstation.
All MR images were reviewed by a stroke neurologist and an interventional neuroradiologist for the presence of ischemic lesions. If diffusion-weighted imaging abnormalities were seen, their number, location, and size were recorded.
Results
Forty-six patients were referred to the interventional neuroradiology service for GDC embolization of cerebral aneurysms between November 1998 and October 1999. Fourteen of the 46 patients were treated for unruptured aneurysms and were enrolled in the study. Ten patients were women and four were men, with a mean age of 57.2 years (age range, 36–73 years). Comorbid conditions included hypertension (three patients), hypercholesterolemia (one patient), and smoking (one patient). There were no other cardiovascular risk factors. Aneurysm locations were as follows: ophthalmic artery (one patient), internal carotid artery paraclinoid (six patients), superior hypophyseal artery (four patients), and anterior communicating artery (three patients). Nine of the aneurysms were <1 cm, and of the remaining five, three were 1 cm and two were large (Table). Aneurysms were discovered during imaging performed for the evaluation of a change in behavior (two patients); headache (six patients, two of whom had aneurysm growth at follow-up); transient ischemic attack workup (four patients); and familial screening (one patient). Additionally, one patient had unruptured aneurysms that were discovered during hospital admission for treatment of subarachnoid hemorrhage from a different source. The Table summarizes the clinical and radiologic findings for each patient.
Reasons for treatment included prevention of subarachnoid hemorrhage and palliation of symptoms in two patients. All patients were first evaluated for open surgical treatments. Endovascular treatment was chosen over surgery because of a higher estimated surgical risk related to aneurysm location, aneurysm morphology (calcific neck, aneurysmal thrombus), poor medical condition, or because of patient choice.
Following the descriptive system presented by Roy et al (10) for aneurysm remnants after coiling, three patients had complete angiographic aneurysm lumen obliteration. In each of three patients, the aneurysmal sac showed a dog-ear neck remnant; in each of three patients, there was a neck remnant; and in five patients, there was residual aneurysm filling (10) (Table). No angiographic evidence of thromboembolism or parent vessel thrombus formation was seen for any of the aforementioned patients. One patient with an anterior communicating artery aneurysm had only 50% of her aneurysm occluded after intraprocedural coil stretch, fracture, and herniation into the internal carotid artery, complicated by a large middle cerebral artery distribution stroke.
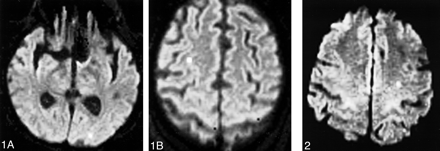
Diffusion-weighted MR imaging showed areas of restricted diffusion with corresponding low apparent diffusion coefficient, most consistent with acute embolic cerebral injury, in nine of 14 patients. All except one of the areas were located ipsilateral to the side of the catheterization. No evidence of small metallic fragments was found on any of the MR sequences. The patient with bilateral injuries had undergone a four-vessel angiogram 4 days before undergoing the coiling procedure (Fig 1A and B); presumably, her contralateral infarct occurred at the time of her diagnostic study. Six patients had evidence of multiple injuries (Fig 2), with all (except one) located in the territory of the vessel catheterized to allow access to the aneurysm. Of the patients with multiple injuries, three patients had two, one had three, one had six, and one with significant complications during the procedure had >10 injuries. All except one of the lesions were small (<2 mm). In the patient with numerous infarcts, therapy was complicated by coil stretch and fracture. Many of her lesions were >2 mm, with a dominant posterior frontal infarct. The use of a balloon-assisted neck-remodeling technique did not seem to increase the incidence of acute embolic cerebral injury. Diffusion-weighted imaging abnormalities were found for three of five patients treated with this technique.
Diffusion-weighted MR images show two small (<2 mm) areas of injury. The patient, a 69-year-old woman, underwent coiling of a 5-mm left paraclinoid aneurysm 4 days after undergoing four-vessel diagnostic angiography. The aneurysm was 95% occluded with four coils.
A, Left hemisphere.
B, Right hemisphere.
fig 2. Diffusion-weighted MR image shows two small (<2 mm) areas of injury in the left hemisphere after the coiling of a 10-mm left paraclinoid aneurysm. The aneurysm was 95% occluded with seven coils, and balloon remodeling was used. The patient's National Institutes of Health Stroke Scale score was 0 before the procedure and was unchanged after the procedure
The National Institutes of Health Stroke Scale scores were unchanged for 13 of 14 patients. For 11 patients, the pre- and postprocedure score was 0. Two patients with baseline deficits (one with a giant [8 cm] anterior communicating aneurysm and the other with previous subarachnoid hemorrhage) were unchanged after GDC therapy, with scores of 6 and 3, respectively.
The patient who suffered the complication of coil stretch and fracture had worsening of her National Institutes of Health Stroke Scale score from 0 to 7. She was discharged to a rehabilitation facility with severe left hemiparesis. All other patients were discharged home.
Overall, the rate of asymptomatic emboli was 61% (eight of 13 treatments) in uncomplicated treatments. Cerebral injuries occurred independently of the number of coils used; the mean number of coils used for patients with infarcts was 7.6 (range, two to 13) and for patients without evidence of infarcts was 10.2 (range, one to 30). This was not a significant difference (P > .5).
Discussion
Significant advances have been made in endovascular techniques for the treatment of intracranial aneurysms (2–4, 11–13), resulting in wide acceptance of GDCs for treatment of saccular intracranial aneurysms. The safety and efficacy of this technique have been well documented (2, 5). Treatment-related complication rates for ruptured and unruptured aneurysms have been reported to be similar or less than those in surgical series. Morbidity figures vary between 3.7% and 10.6%, with mortality between 1% and 2.3% (14, 15).
Complications during GDC procedures have several possible causes. As an angiography-based procedure, GDC therapy shares the risks of routine diagnostic cerebral angiography. Hemostatic control at the puncture site, large vessel dissection, contrast reactions, renal failure, and infection probably occur at rates similar to diagnostic cerebral angiography and account for only a minority of the morbidity and mortality rates. The mechanical forces of navigating a microcatheter through the arteries and into the aneurysm or from placing the GDCs into the aneurysm can result in trauma to the vessel wall and loss of integrity. Fortunately, perforations of aneurysms during embolization are uncommon and many perforations can be successfully sealed before irreversible injury occurs. Nevertheless, perforations and intraprocedural aneurysm ruptures can be important contributors to procedural mortality. Mechanical factors related to navigation and coil placement can also be responsible for other types of complications, such as parent artery dissection; impingement on adjacent branches or perforators; stretching, fracture, or migration of coils; and vasospasm.
The largest contributor to morbidity and mortality rates in GDC therapy has been iatrogenic brain ischemia. Although brain ischemia can be caused by complications such as inadvertent vessel occlusion from coil migration, coil rupture, or other type of failure, impingement by coils, dissection, or severe vasospasm, thromboembolic phenomena are presumed to account for a major proportion. The frequency of thromboembolic complications, including stroke and transient ischemic attacks, has been reported to be between 3.2% and 28% (6, 16). The majority of these complications are transient, lasting <48 hours. Until recently, the diagnosis of a thromboembolic event has largely relied on changes in the results of neurologic examinations with no direct examination of the emboli or the associated brain injury. Recently, continuous transcranial Doppler monitoring has been used during endovascular procedures to detect microemboli (7). Microemboli were seen more frequently in patients who suffered clinically evident cerebral ischemia (mostly transient) after coil embolization than in asymptomatic patients. This information seems to support the hypothesis that many of these transient neurologic deficits are caused by microembolic events.
There are multiple theoretical sources for embolic events during GDC aneurysm therapy. Friable plaque in the parent vessel can be dislodged with resultant distal embolization; an iatrogenic dissection of a vessel can provide a source of thrombus; preexisting thrombus or fresh clot within an aneurysm can migrate distally during coil placement; and thromboemboli can form on the catheter or coils. Microemboli are not necessarily related to clot but can be metallic fragments from the GDCs (17), and air bubbles in the infusion solutions may act as emboli. With increasing procedural complexity, the potential sites for the generation of microemboli increase, and, in combination with longer procedure times, the risk of significant emboli will likely increase. For GDC procedures, larger aneurysms requiring larger numbers of coils or the use of a double-catheter technique or balloon-remodeling technique theoretically increases the risks of thromboembolic complications. With the use of balloon remodeling, low flow during occlusion can contribute to thrombus formation, and the balloon itself may function as a thrombotic nidus.
These statements are largely a matter of theoretical discussion because no direct study of the incidence and effects of thromboembolic phenomena has been performed. The ability to measure these effects accurately is critical to further refinement of embolization techniques, because it provides a means of comparing changes in the technique.
The initial step in this process is to determine the baseline rate of embolic events by use of a standardized protocol. Diffusion-weighted MR imaging was used in our study to clarify the incidence, size, and location of acute stroke after coiling procedures and was achieved using our standardized protocol. We chose this imaging technique because diffusion-weighted imaging is highly sensitive and specific in diagnosing acute ischemic stroke and early ischemia (9, 18). In animal experiments, diffusion-weighted imaging reveals abnormal results within 30 minutes in an ischemic zone, whereas in humans, diffusion-weighted imaging reveals abnormal results within 40 minutes of the onset of ischemic stroke. Considering this technique's accuracy in identifying early ischemic injury, it is likely that only the smallest injuries were missed in our patient cohort.
Our study is not without limitations, including its relatively small cohort of patients and the absence of a preprocedural baseline MR imaging study. However, none of the study patients had any clinical history of acute neurologic events in the days preceding the GDC procedure. In addition, the T2-weighted imaging sequences did not show parenchymal abnormalities corresponding to those seen on diffusion-weighted images, which indicates that the possibility of these strokes having occurred before the procedure is unlikely.
We were able to show that small, clinically asymptomatic infarcts occur frequently during the GDC procedure. The pattern of brain injury is consistent with small microemboli causing small vessel occlusions. The MR imaging did not reveal metallic artifacts to support metallic fragments as the cause of the emboli. They are almost certainly thromboembolic phenomena, although air emboli may also contribute. In two patients, infarcts were identified in vascular territories other than the one catheterized for the GDC procedure. Each of these patients had undergone recent diagnostic cerebral angiography that may have been the source of these emboli. Certainly, as an angiographic procedure, GDC therapy has a diagnostic component as well as a therapeutic one. So, the rate of embolic events would predictably be higher than with diagnostic angiography alone. This is borne out in the study by Bendszus et al (19) that found the rate of silent embolism in diagnostic cerebral angiography by MR imaging to be 26%, which is significantly lower than our rate of 54%.
It is not surprising that the injuries revealed by MR imaging are clinically silent, considering the relatively small amounts of brain affected. Similarly, these are unlikely to cause abnormalities on neuropsychological testing. The location of the lesion depends on the flow patterns in the patient's brain and on chance. If an eloquent region of brain were involved, lesions of this size could cause a significant clinical syndrome, but this did not occur in any of our patients. This may well account for the relatively high rates of transient neurologic deficits reported in previous series (6, 16).
Our series includes one patient with a symptomatic event corresponding to a mechanical complication from a coil fracture and embolus. Our rate of symptomatic complications falls within the range reported in previous series (5, 6). However, our rate of clinically silent emboli is not comparable with those of previous series. Murayama et al (5) found no cases of thromboembolic complications among 115 patients; only clinical complications were recorded. Pelz et al (6) found a symptomatic thromboembolic rate of 28% in their series. This series included not only patients with asymptomatic aneurysms but also patients with subarachnoid hemorrhage. In addition, anticoagulation was not rigorously used during all their procedures. Our series maintained a standardized protocol for the use of anticoagulation and, by including only elective aneurysms, attempted to isolate procedurally related events from those caused by other clinical factors, such as subarachnoid hemorrhage. We routinely continue systemic anticoagulation for 2 days after the procedure has been performed to prevent late embolic events. This level of anticoagulation is the one used at our institution to prevent recurrent ischemic embolic strokes. The high rate of events identified by MR imaging in our study argues that our protocol for systemic anticoagulation may not be adequate, and that we should consider adding antiplatelet agents for prevention of embolic events.
Currently, we have been using antiplatelet agents after the discontinuation of systemic anticoagulation (heparin) to limit the risk of intracerebral and systemic bleeding. However, prospective studies with long-term follow-up are necessary. In such studies, diffusion-weighted MR imaging could be used as an objective measure of microembolic events.
Conclusion
We have documented a relatively high prevalence of small, clinically asymptomatic, thromboembolic events that occurred during or immediately after otherwise uneventful GDC embolization of intracranial aneurysms. However, clinical sequelae are rare. Measures to reduce the rate of silent and symptomatic emboli may permit safer aneurysm treatment in the future.
Footnotes
↵1 This work was presented at the Joint Meeting of the AANS/CNS Section on Cerebrovascular Surgery and the American Society of Interventional and Therapeutic Neuroradiology, February 6–9, 2000, New Orleans.
References
- Received March 10, 2000.
- Copyright © American Society of Neuroradiology