Abstract
BACKGROUND AND PURPOSE: Optic pathway and/or hypothalamic astrocytomas in children are often quiescent, but in some cases, more aggressive tumors may cause progressive visual, endocrine, and neurologic deterioration. The initial treatment of these gliomas includes surgery and IV chemotherapy. Radiotherapy is not recommended in young children because of its severe adverse effects on cognitive and neuroendocrine function. This report suggests a new approach using combined intraarterial and IV carboplatin-based chemotherapy for patients for whom first line treatment has already failed.
METHODS: Six children (mean age, 57 months) with the diagnosis of optic pathway hypothalamic gliomas, who had tumor progression after surgery and underwent IV chemotherapy, were treated monthly with intraarterially administered carboplatin, intraarterially administered etoposide phosphate, and IV administered Cytoxan. Four of the children had histologically verified pilocytic astrocytomas, and in two cases, diagnosis was made on the basis of clinical findings. Administration of the intraarterial chemotherapy required catheter placement in both internal carotid arteries at the level of C2–C3 and into one of the vertebral arteries at the level of C6–C7, with the patient under general anesthesia.
RESULTS: Four of six patients had partial radiographic response, one had stable disease, and one had progressive disease after one cycle. Three patients showed clinical improvement. There were no serious complications associated with the angiographic procedures. Toxicities included bronchospasm that resolved after 3 to 4 minutes in one patient. One patient showed mild ototoxicity, and four patients needed platelet transfusion because of hematologic toxicity of drugs.
CONCLUSION: These results suggest that this modality of chemotherapy (administered after failure of systemic [ie, IV] chemotherapy), of progressive optic-hypothalamic astrocytomas in young children may be an effective treatment prior to radiotherapy.
Optic-chiasm and/or hypothalamic gliomas have been considered benign tumors because of their histologic appearance; however, the biologic behavior of juvenile pilocytic astrocytomas in this location is age-dependent. In patients younger than 5 years, these tumors often exhibit aggressive growth and recurrence after surgical resection and/or IV chemotherapy treatment (1).
Several authors have made recommendations for the management of these tumors (2–5). The most common current treatment strategy in patients with or without neurofibromatosis type 1 with a rapidly growing suprasellar lesion associated with visual deterioration or progressive neurologic deficits is surgery. In contrast to juvenile pilocytic astrocytomas in the posterior fossa, which are surgically resectable, tumors located in the suprasellar region are only partially resectable. The residual tumor often shows progression on serial neuroimages. Patients with progressive disease whose vision is significantly impaired receive systemic (ie, IV) chemotherapy. Unfortunately, after a period of good response, the lesions often progress. At the time of further progression, radiation therapy is considered for children older than 5 years but is not recommended for younger children, primarily because of neurocognitive complications (2, 6, 7). In addition, especially in patients with neurofibromatosis type 1, cerebrovascular complications occur more often after radiotherapy (6, 8). One of the most recent studies noted an incidence of radiation-induced cerebral vasculopathy as high as 30% in children with neurofibromatosis type 1 (9). Some studies report the development of different types of endocrine abnormalities, such as growth hormone deficiency and hypogonadotrophic hypogonadism, after field irradiation (2, 10). Considering the high complication rate of radiotherapy in young patients, other therapeutic options should be considered to delay irradiation. Although gamma knife radiosurgery has been suggested (11), further experience with this treatment modality is needed, including long-term follow-up to evaluate potential side effects. Also, radiosurgery is not available to all patients, and visual complications can occur.
Six children with optic pathway and chiasmatic-hypothalamic gliomas were treated with combined intraarterial and IV chemotherapy at Oregon Health Sciences University between September 1996 and October 1999. This report describes the response in patients treated with intraarterial chemotherapy for whom the previous treatment of partial tumor resection and then IV chemotherapy had failed (12–17). The results suggest that intraarterial chemotherapy is feasible in young children and show this to be an active second line treatment for chiasmatic-hypothalamic astrocytomas before radiation.
Methods
Patient Selection
Between September 1996 and October 1999, six children with progressing low-grade chiasmatic-hypothalamic astrocytomas were treated at Oregon Health Sciences University with combined intraarterial and IV chemotherapy. Previous treatment of partial tumor resection and IV chemotherapy had failed for these patients. The patients were referred to us by a pediatric oncologist because, despite the previous treatments, the disease remained progressive. Four of the patients had histologically verified diagnoses, and in two cases, diagnosis was made on the basis of clinical findings. All patients had been treated with varying regimens of chemotherapy, but none had received previous radiotherapy. Before treatment with combined intraarterial and IV chemotherapy at Oregon Health Sciences University, MR imaging was performed and revealed marked tumor progression.
Treatment
The treatment protocol consists of intraarterially administered carboplatin, intraarterially administered etoposide phosphate, and IV administered Cytoxan every 4 weeks, for up to 12 courses. Carboplatin (400 mg/m2) and etoposide phosphate (400 mg/m2) were administered over 10 minutes in equally divided doses in the right and left internal carotid arteries and one of the vertebral arteries. Cytoxan (660 mg/m2) was IV administered over 15 minutes simultaneously with the intraarterially administered drugs. For selective catheterization of the internal carotid and vertebral arteries, a transfemoral approach and standard catheter and guidewire technique were used. The catheter was placed at the C2–C3 level for internal carotid artery injections and at the C6–C7 level for vertebral artery injections. The procedure was performed with the patient under general anesthesia. Before each course of chemotherapy, all patients underwent tests for complete blood cell count, electrolytes, and liver and renal function, coagulation studies (prothrombin time, partial thromboplastin time), chest radiography, and CT or MR imaging. Baseline audiologic assessment was also conducted. All patients were monitored in the pediatric intensive care unit for 24 hours after the procedures, especially regarding their fluid balance, vital signs, and temperature. Granulocyte colony-stimulating factor was administered with all courses, starting 48 hours after the last dose of chemotherapy, for 7 to 10 days. Chemotherapy dosages were modified in subsequent cycles for myelosuppression or audiologic toxicity. When the platelet nadir was <20,000/mm3 or neutropenic fever with positive blood culture or high frequency hearing loss occurred, the carboplatin dose was reduced by 25% for all subsequent courses.
Standard radiographic criteria were used to assess tumor response. Partial response was defined as >50% decrease in tumor volume for at least 4 weeks, stable disease was defined as <25% increase in volume, and progressive disease was defined as >25% increase in tumor volume. Responses were evaluated by contrast-enhancing CT or T1-weighted MR imaging by measuring the anteroposterior, the latero-lateral, and the craniocaudal diameters of the tumor. Clinical response was assessed by neurologic, ophthalmologic, endocrinologic, and psychologic examinations.
Patient Characteristics
The most important characteristics of our six cases are summarized in the Table. The mean patient age at the time of diagnosis was 19.3 months (range, 1–80 months) and at the start of intraarterial treatment was 57 months (range, 27–81 months). One patient underwent direct partial resection, and three patients underwent initial biopsy for the purpose of obtaining definitive histology. Two of these three patients later also underwent surgery because of tumor progression. All six patients had already received chemotherapy, and five of the six patients had previously received more than one series of IV chemotherapy. The most commonly used drugs were carboplatin, vincristine, etoposide, procarbazine, lomustine, and 6-thioguanine.
Treatment summary and outcomes
Four of the six patients had nystagmus or other disorders of eye movement and vision, and two had diencephalic syndrome at onset of the disease. Two patients were diagnosed with neurofibromatosis type 1, one at the time the clinical diagnosis was made and the other later on in the course of the disease. For one patient, MR imaging of the spine revealed leptomeningeal dissemination. All patients except one developed hydrocephalus within a few months after diagnosis. These patients required placement of a ventriculoperitoneal shunt. Five of the six patients experienced neutropenia/neutropenic fever, sepsis, or severe thrombocytopenia during the preceding systemic chemotherapy.
Case Studies
Case 1
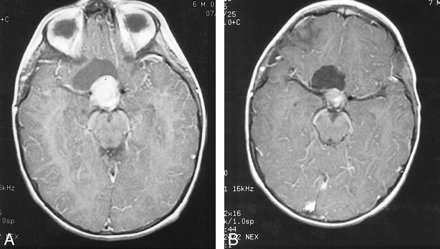
A 10-month-old male patient with right eye nystagmus was diagnosed by CT to have a partly cystic, partly solid, contrast-enhancing chiasmatic glioma. The patient underwent partial excision with histopathology of pilocytic astrocytoma and was treated with carboplatin and etoposide for four cycles. Eight months later, CT revealed tumor progression, and the patient was treated with vincristine and lomustine for a year. At that time, his right eye showed amblyopia and total optic atrophy. One year later, the disease had again progressed, and lomustine, procarbazine, and 6-thioguanine was administered for another year. Fourteen months later, evidence of progression was again revealed by MR imaging (Fig 1A) and visual field examination showed significant visual field loss in the left eye. At that time, the patient underwent combined intraarterial and IV chemotherapy. After the first procedure, the patient's platelet nadir was 15,000/mm3. For subsequent cycles, the carboplatin dose was reduced by 25%, and two further transfusions were required after the seventh and 12th treatments. After four courses, the visual fields showed moderate improvement and the monthly CT scans showed moderate but continuous decrease in the size of the contrast-enhancing region of the tumor. After completion of 12 cycles of chemotherapy, eye examination revealed improvement in visual fields. Three months after completion of chemotherapy, MR imaging showed partial response with further reduction in the solid part of the tumor (Fig 1B).
Images from the case of a 10-month-old male patient with right eye nystagmus who was diagnosed by CT to have a partly cystic, partly solid, contrast-enhancing chiasmatic glioma (case 1).
A, Contrast-enhanced axial view T1-weighted MR image, obtained after partial resection and three regimens of systemic chemotherapy, shows a strongly contrast-enhancing nodular suprasellar mass with an associated cystic component.
B, After 12 courses of combined intraarterial and IV chemotherapy, the volume of the solid tumor component and the degree of contrast enhancement are significantly decreased. There is no significant change in the volume of cystic component
Case 2
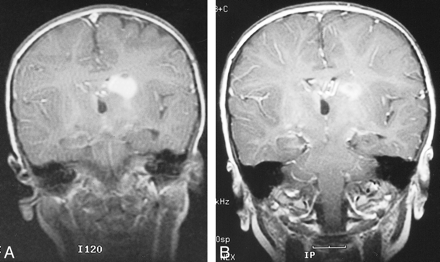
A 3-week-old female patient presented with bilateral rotatory and lateral nystagmus. MR imaging revealed diffuse optic-hypothalamic glioma and hydrocephalus. A biopsy was performed and ventriculoperitoneal shunt placed. The biopsy verified juvenile pilocytic astrocytoma. The patient completed eight courses of carboplatin and vincristine chemotherapy and initially had a good response, but therapy was associated with significant neutropenia. One year later, MR imaging revealed disease progression, and the patient underwent treatment with vincristine, 6-thioguanine, procarbazine, and lomustine. After four cycles, MR imaging showed tumor progression (Fig 2A) and combined intraarterial and IV chemotherapy was begun. After the first course, the patient required a platelet transfusion and had hemorrhagic cystitis, which resolved quickly with hydration. Subsequently, with each treatment, the carboplatin dose was reduced by 25%. The contrast-enhancing tumor decreased significantly after six courses (Fig 2B). Two treatments were complicated by bronchospasm with accompanying oxygen desaturation, which was well controlled by anesthesiology. The possibility of an allergic reaction against the intraarterial drugs was excluded with appropriate testing. Although the patient was developmentally delayed at the beginning of the combined chemotherapy, some improvement in her movement, strength, and speech occurred. The patient continued to receive therapy, with a partial response being achieved after eight cycles.
Images from the case of a 3-week-old female patient who presented with bilateral rotatory and lateral nystagmus (case 2).
A, Coronal view T1-weighted MR image reveals a solid contrast-enhancing lesion in the territory of the left basal ganglia and corona radiata after two regimens of IV chemotherapy.
B, After six courses of combined intraarterial and IV chemotherapy, there is a significant decrease in the tumor volume and contrast enhancement
Case 3
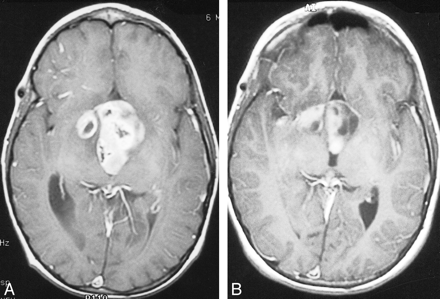
A 6.5-year-old male patient presented with weight loss and cerebral salt-wasting syndrome. This patient was diagnosed with neurofibromatosis type 1 and a large diencephalic tumor with hydrocephalus. There was no family history of neurofibromatosis. The patient underwent ventriculoperitoneal shunt placement. Two months later, he was noted to have decreased visual acuity and MR imaging revealed tumor progression (Fig 3A). A regimen of low-dose orally administered etoposide and steroids was begun. Because of the fast growing tumor, the next month, the patient was treated with combined intraarterial and IV chemotherapy. At the time of first treatment, neutropenic fever developed during a prolonged hospital course necessitated by cerebral salt-wasting syndrome. The patient underwent 11 further courses of chemotherapy without complication. Follow-up MR imaging showed partial tumor response (Fig 3B). The ventriculoperitoneal shunt was revised several times, and because of cerebral salt-wasting syndrome, electrolytes were closely monitored. Although the intake of oral sodium was necessary, the patient remained in good condition for more than 1.5 years, until his vision decreased in September 1999. MR imaging revealed some increase in tumor size, so the patient started his second series of combined intraarterial and IV chemotherapy in October 1999.
Images from the case of a 6.5-year-old male patient who presented with weight loss and cerebral salt-wasting syndrome (case 3).
A, Axial view T1-weighted MR image, obtained after one course of orally administered chemotherapy, shows an inhomogeneously contrast-enhancing large mass extending into the third ventricle and right temporal lobe. Posteriorly, the tumor fills the entire lumen of the ventricle.
B, Follow-up contrast-enhanced MR image, obtained 6 months after completing 12 courses of combined intraarterial and IV chemotherapy, shows marked shrinkage of the contrast-enhancing lesion (notice the free part of the dorsal third ventricle). A more cystic component is present within the left side of the tumor
Results
Six children with progressive chiasmatic-hypothalamic low grade gliomas were treated with combined intraarterial and IV chemotherapy after previous treatment with other chemotherapy regimens had failed. Three of the six patients also had undergone previous surgery. Despite previous surgery and systemic chemotherapy, the tumors showed progression. Subsequent combined intraarterial and IV chemotherapy halted progression in five of the six cases. The Table summarizes the radiologic results, clinical outcomes, and complications. Three of six patients (patients 1, 2, and 3) achieved partial response with clinical improvement, and one patient (patient 6) achieved partial response with mild hearing loss after five courses. Patient 4 achieved a short period of minimal response, and the disease then progressed. Patient 5 had stable disease with stable clinical status until ventricular hemorrhage and death occurred before the fifth course. In general, this chemotherapy regimen was well tolerated, with the exception of two treatments in one patient in whom bronchospasm occurred during intraarterial drug administration. The toxicity of the chemotherapeutic agents was variable. Four of six patients had platelet nadirs below 20,000/mm3, which required prophylactic platelet transfusion one time in two patients, three times in one patient, and monthly in the fourth patient. For three of the four patients, the dose of carboplatin had been reduced by 25%, and for one patient, all drug doses had been reduced by 50%. Episodes of neutropenia/neutropenic fever requiring hospitalization occurred only three times during 46 courses of intraarterial chemotherapy, one with accompanying sepsis. High frequency hearing loss, most likely due to intraarterial carboplatin, occurred in one patient. Renal insufficiency did not occur. One patient had hemorrhagic cystitis; however, this was mild and treatable. During the short follow-up period, no neurologic, ophthalmologic, or endocrinologic deteriorations were observed except in patient 3, who had progressive loss in visual acuity after 33 months.
Discussion
The initial treatment of visual pathway and hypothalamic gliomas is controversial (2), and when tumor progression has occurred, treatment options become even more complex. Although surgical resection is usually not a viable option for definitive treatment of these tumors, recent studies have shown favorable results with additional systemic chemotherapy (5, 12), especially in young patients who are at risk for irradiation-induced adverse sequelae. A wide variety of agents have been used (13–17), but the optimal combination of chemotherapeutic drugs remains under investigation. Several authors emphasize the efficacy of platinum-based agents in combination with vincristine (12–14) against recurring or progressing optic-hypothalamic gliomas. Others have studied carmustine, lomustine, 6-thioguanine, etoposide, or procarbazine in combination with other drugs (2, 15–17). Although these drugs prove to be effective in most patients, some patients show disease progression during or after completion of systemic chemotherapy.
In the current series, the total dose of intraarterially administered chemotherapy agents was either the same or less than the doses administered previously to these patients. Intraarterial administration may increase drug concentrations intracranially two- to 10-fold, decreasing the need for high dose IV chemotherapy and the associated increase in systemic toxicity (18–20). Systemic chemotherapy, particularly with regimens such as procarbazine, lomustine, and vincristine, is associated with significant acute toxicities, including nausea/vomiting, severe and progressive myelosuppression, and risk of infection. Chemotherapy administered by intraarterial infusion may decrease the extent of systemic exposure while maximizing anti-tumor effect in the CNS. On the other hand, when moderate myelosuppression is present, necessitating a reduction of drug dose, the remaining 50% to 75% dose has still proved effective when administered intraarterially. The transfemoral approach for selective intraarterial drug administration is a safe procedure with a very low complication rate (19).
Aquino et al (21) reported the cases of 12 patients with optic chiasm glioma treated with IV administered carboplatin. Comparing the present study with that presented by Aquino et al, the current series used stricter criteria to determine partial response. If the same criteria were used for partial response as those used in the report by Aquino et al (“regression of disease not meeting the criteria for complete response” [ie, any decrease in tumor size]), patient 4 would also have achieved a short period of partial response after changing from carboplatin to methotrexate. The average overall dose administered intraarterially in the current series was lower than that reported by Aquino et al. For instance, etoposide (400 mg/m2) was administered as a single dose, but the total dose was lower than the usual IV course (100 mg/m2 for 5 days). The patients in the study presented by Aquino et al, in contrast to the patients in the current series, had not undergone any previous chemotherapy when they received IV administered carboplatin as the initial therapy. All except one of our six patients had already undergone several courses of chemotherapy, including IV administered carboplatin, and their disease had progressed. Most of our patients were thereby perhaps already partially resistant to IV administered carboplatin, but they showed good response when this drug was administered intraarterially to increase CNS drug delivery.
The monthly cost for anesthesia and catheter placement are increased, but the alternative of cognitive sequelae with radiation to this area of brain or high dose chemotherapy with bone marrow rescue is also costly. Since 1981, intraarterial chemotherapy has been used with or without blood-brain barrier disruption at Oregon Health Sciences University to treat a variety of brain tumors, including primary CNS lymphomas, primitive neuroectodermal tumors, gliomas, metastasis, and germinomas (18, 20). Carboplatin as a platinum-based chemotherapy drug has a proved effect in both malignant brain tumors and more benign gliomas, such as juvenile pilocytic astrocytomas. Etoposide phosphate in combination with carboplatin has a synergistic antitumoral effect when administered intraarterially. Cytoxan, IV administered (to allow hepatic activation), is another alkylating agent and DNA synthesis inhibitor.
This tri-drug regimen proved effective in controlling tumor progression in five of six patients with juvenile pilocytic astrocytomas who were previously treated with systemic chemotherapy. Because of the short follow-up duration, long-term efficacy is not available. However, this regimen may effectively delay radiotherapy, which is advantageous with respect to preserving long-term intellectual and endocrine function and avoiding radiation-induced cerebral vasculopathy in young children with neurofibromatosis type 1 (9).
The accompanying systemic toxicity proved to be less by intraarterial administration of drugs compared with the larger systemic doses of chemotherapy necessary to attain equivalent CNS tumor drug levels. The result is a better quality of life between treatments.
Conclusion
These preliminary results suggest that the tri-drug chemotherapy regimen of combined intraarterially administered carboplatin, intraarterially administered etoposide phosphate, and IV administered Cytoxan may be an effective and safe treatment modality for progressive optic-hypothalamic gliomas in young children before radiotherapy. However, further follow-up and larger series of patients are required for evaluation of long-term efficacy and comparison with other treatment modalities and combinations of different drugs is necessary in the future.
Footnotes
1 This research was funded by a Veterans Administration Merit Review Grant, grant CA31770 from the National Cancer Institute, and grants NS34608 and NS33618 from the National Institutes of Neurological Disorders and Stroke.
↵2 Address reprint requests to Edward A. Neuwelt, MD, Oregon Health Sciences University, Department of Neurology, 3181 SW Sam Jackson Park Road, L603, Portland, OR 97201.
References
- Received January 13, 2000.
- Copyright © American Society of Neuroradiology