Abstract
Summary: Neurocutaneous melanosis (NCM) is a rare neuroectodermal dysplasia characterized by large or multiple cutaneous congenital pigmented nevi and benign or malignant melanocytic tumors of the leptomeninges. Although the MR manifestations of this disease have been reported in a small series of cases, the usefulness of fluid-attenuated inversion recovery (FLAIR) MR findings has not been documented. We present a case of NCM that showed diffuse leptomeningeal hyperintensity on FLAIR images. This FLAIR finding may be a clue to the detection of leptomeningeal abnormalities in NCM.
Neurocutaneous melanosis (NCM) is a rare congenital disease characterized by the presence of large or multiple congenital melanocytic nevi and benign or malignant melanocytic tumors of the leptomeninges (1). Although about 100 cases of NCM have been described in the literature, there have been only a few reports concerning the MR findings of this disease, including high signal intensity melanosis of the temporal lobe on T1-weighted images (2–4) and abnormal leptomeningeal enhancement with benign melanocytic proliferation (5–7) or with malignant melanoma (8, 9). We present a patient with NCM who demonstrated diffuse leptomeningeal hyperintensity on fluid-attenuated inversion recovery (FLAIR) images.
Case Report
An 8-year-old Japanese boy was admitted to our hospital with a 4-month history of headache, vomiting, and convulsions. Neurologic examination revealed impaired visual acuity and partial visual field defects. Meningeal signs such as stiff neck and the Kernig sign were observed. Laboratory findings were unremarkable. His medical history was notable for a solitary, large (maximum diameter of 7 cm) hairy nevus on his back at birth. His neurologic development was normal before onset of current symptoms.
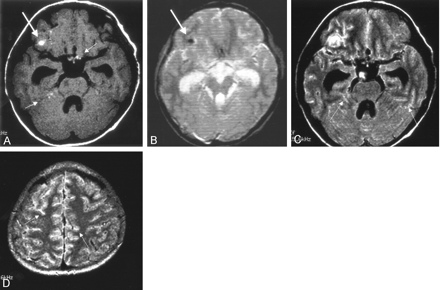
CT scanning showed hydrocephalus and a hyperattenuated lesion in the right frontal lobe. No other abnormal findings were observed with CT. MR imaging at 1.5 T showed high signal intensity on T1-weighted images (Fig 1A) and very low signal intensity on T2*-weighted images (Fig 1B) in the corresponding right frontal lobe region. Multiple high signal intensity lesions were also seen on the surface of the brain on T1-weighted images (Fig 1A); however, no decrease in signal intensity was observed in these lesions on T2*-weighted images. FLAIR images showed diffuse leptomeningeal hyperintensity (Fig 1C and D). Contrast-enhanced imaging was not performed, because the patient could not tolerate further MR study. Lumbar puncture was performed 5 days after MR examination and showed extremely high CSF pressure. The CSF was clear, the cell count in the CSF was slightly elevated (18 cells/μL), the glucose level was 99 mg/dL (with a blood glucose level of 168 mg/dL), and the protein level in the CSF was markedly elevated (462 mg/dL). The findings of microbiological studies for bacteria, viruses, and fungi were all negative. Analysis of CSF samples suggested the presence of melanocytes with no evidence of malignancy. Although leptomeningeal abnormalities were strongly suspected on the basis of the results of MR examination, as well as the patient’s history, no diagnosis was established.
Initial MR imaging 3 days after the CT study.
A, T1-weighted image (616/14) shows a high signal intensity focus in the area of the right frontal lobe (arrow). Other high signal intensity foci are seen at multiple sites (small arrows).
B, T2*-weighted image (GRE, 400/20/FA 20) shows a low-signal intensity focus in the corresponding area of the right frontal lobe (arrow), which was confirmed as hemorrhage at surgery. No other low signal intensity lesions are seen.
C and D, FLAIR images (8002/133/2000) show diffuse leptomeningeal hyperintensity in the sulci (small arrows).
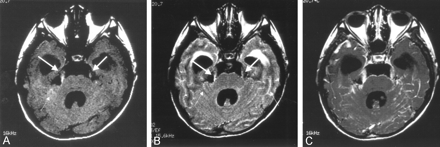
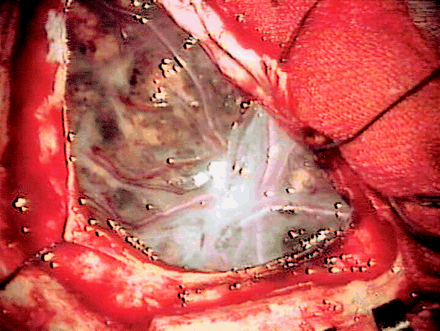
The patient lost consciousness and developed progressive muscle weakness of the upper and lower extremities. A second MR study was performed 3 weeks after the initial one. MR imaging showed progressive hydrocephalus with diffuse leptomeningeal hyperintensity on FLAIR images (Fig 2B) and diffuse leptomeningeal enhancement in the brain and spine on contrast-enhanced T1-weighted images (Fig 2C). At this point, leptomeningeal metastasis from a malignant tumor of unknown origin was suspected. Surgery was performed to relieve hydrocephalus and to obtain pathologic specimens from the leptomeninges in the right frontal region. At surgery, diffuse black pigmentation of the leptomeninges was found (Fig 3). The hematoma in the right frontal lobe was adjacent to the leptomeningeal tumor and was likely to derive from the tumor. Biopsy was performed at multiple sites of the pigmented leptomeninges. Pathologic examination showed malignant melanoma of the leptomeninges (Fig 4). Results of the skin biopsy were consistent with a congenital nevus without a malignant component. On the basis of these findings, the final diagnosis was neurocutaneous melanosis.
Follow-up MR imaging 3 weeks after the initial MR study.
A, T1-weighted image (616/14) shows high signal intensity in both trigeminal nerves (arrows). High signal intensity foci are also seen in the cerebellar sulci (small arrow). Note that hydrocephalus has progressed.
B, FLAIR image (8002/133/2000) shows diffuse leptomeningeal hyperintensity (small arrows). Both trigeminal nerves are thickened and hyperintense (arrows).
C, Contrast-enhanced T1-weighted image (616/14) shows diffuse leptomeningeal enhancement corresponding to the findings of FLAIR imaging.
Appearance at surgery. Diffuse black pigmentation of the leptomeninges is noted.
A, Photomicrograph (hematoxylin and eosin stain, × 400) shows the atypical cells with dark melanin pigment. The mitotic figures were frequently observed in the neoplasm.
B, Photomicrograph shows a strongly positive immunohistochemical reaction to antimelanoma antibody (HMB-45).
The patient died of intracranial hemorrhage 6 months after initial presentation. His family refused autopsy.
Discussion
NCM is a rare type of phakomatosis, although it probably represents the most common form of intracranial malignant melanoma in the pediatric population. The disease is thought to be a form of embryonal neuroectodermal dysplasia. Fox (10) did much of the early work on this disease, and he proposed the original diagnostic criteria in 1972 (11). Kadonaga and Frieden (1) proposed the following revised criteria: large (is or is estimated to become no less than 20 cm in diameter in adults/6–9 cm in infants) or multiple (no less than three lesions) congenital nevi in association with meningeal melanosis or melanoma; no evidence of cutaneous melanoma, except in patients in whom the examined areas of the meningeal lesions are histologically benign; and no evidence of meningeal melanoma, except in patients in whom the examined areas of the cutaneous lesions are histologically benign. On the basis of their criteria, a definitive diagnosis of NCM could be established in our patient.
NCM patients with leptomeningeal involvement have the potential to develop malignant melanoma, with an estimated prevalence of 40–60% (1, 11). Symptomatic leptomeningeal melanoma is usually associated with an extremely poor prognosis (8, 9). Even with benign melanocytic proliferation of the leptomeninges, symptomatic NCM has a poor prognosis (1, 5). Our patient showed progressive neurologic deterioration, with evidence of progressive hydrocephalus and extensive leptomeningeal abnormalities on serial MR images. The rapid growth of leptomeningeal melanoma in a short period was presumably responsible for the rapid increase in intracranial pressure and the repeated episodes of headache, vomiting, and seizures, as well as coma in the final stages.
The MR findings of NCM are variable, including hyperintense areas in the temporal lobe on T1-weighted images without leptomeningeal enhancement (3, 4), diffuse leptomeningeal enhancement of the brain and spine on contrast-enhanced T1-weighted images (5–7, 9), and extensive mass of malignant melanoma (8). Thus, NCM can show a wide spectrum of MR imaging findings. In our case, abnormal hyperintense lesions were seen on T1-weighted images in multiple areas on the brain surface. Although a high signal intensity focus in the right frontal lobe was confirmed to be hemorrhage from melanoma, other high signal intensity foci were likely due to the T1-shortening effect of melanin, because no decrease in signal intensity was observed in these high signal intensity foci (except for the right frontal lesion) on T2*-weighted images (Fig 1B). In our case, the presence of high signal intensity on T1-weighted images was not a prominent finding compared with the appearance of diffuse leptomeningeal abnormalities on FLAIR and contrast-enhanced images. Therefore, T1-weighted images may not be sufficient for detecting melanocytic proliferation of the leptomeninges in NCM.
Although frequent leptomeningeal involvement is an intrinsic characteristic of NCM, such leptomeningeal abnormalities on FLAIR images have not been reported in patients with NCM. Chu et al (9) reported in their patient with NCM that FLAIR images depicted a temporal lobe mass as a high-signal intensity area. They did not, however, describe leptomeningeal hyperintensity on FLAIR images.
A variety of central nervous system diseases are associated with abnormal hyperintensity within the subarachnoid space on FLAIR MR imaging (12), including subarachnoid hemorrhage (13), meningitis (14), leptomeningeal metastases (15), and moyamoya disease (16). The appearance of NCM on FLAIR images is similar to that of the above-mentioned diseases, and it is therefore difficult to differentiate among them on the basis of FLAIR images alone. The mechanisms of the abnormal FLAIR hyperintensity observed in our patient remain unknown, but it is speculated that the high protein concentration in the CSF may be a factor (15). Another possible mechanism is that the T1-shortening effect of malignant melanoma (melanin) may contribute to the hyperintensity seen on FLAIR images. The latter mechanism seems likely because both trigeminal nerves showed thickening and hyperintensity on T1-weighted and FLAIR images and intense enhancement on contrast-enhanced T1-weighted images (Fig 2). The findings on FLAIR imaging were in agreement with those of contrast-enhanced imaging with regard to the delineation of leptomeningeal lesions.
Acknowledgments
We would like to thank Drs. Takao Kodama and Hitoshi Terada, for their invaluable advice in this case.
References
- Received March 28, 2003.
- Accepted after revision April 22, 2003.
- Copyright © American Society of Neuroradiology