Abstract
BACKGROUND AND PURPOSE: Endovascular treatment of wide-necked aneurysms remains a therapeutic challenge. We conducted this study to evaluate the angiographic results and clinical outcome of patients treated with stent-assisted coiling by using a recently available self-expandable intracranial stent.
METHODS: A retrospective review of all patients treated with self-expandable stent-assisted coiling between September 2002 and December 2003 was done. Treatment was attempted in 32 patients with 35 aneurysms. Four of the aneurysms were ruptured. All had either a dome-to-neck ratio less than 2 and/or a neck diameter of 5 mm or larger. Following stent placement, coiling was attempted in 33 of 34 aneurysms. The technical success of the procedure, procedure related complications, and the angiographic results were documented.
RESULTS: In 34 of 35 aneurysms, stent deployment across the neck of the aneurysm was successful. Coiling was performed successfully in 30 of 33 aneurysms. In 20 aneurysms, immediate posttreatment angiography showed either total (17%) or satisfactory (50%) occlusion. Procedure-related mortality occurred in one patient (3.1%). Adverse events occurred in eight patients (25%); in three of them permanent neurologic deficit resulted (9.3%). In six patients, thrombus formation occurred within the stented segments during the procedure and reopro infusion was used. Follow-up angiography was available in 12 (40%) of 30 treated aneurysms.
CONCLUSION: In our practice use of the self-expandable stent seemed to facilitate endovascular treatment of wide-necked intracranial aneurysms. Difficulty of deployment and stent thrombogenicity are the main drawbacks of the system.
Aneurysmal subarachnoid hemorrhage (SAH) is a major health problem, occurring with a frequency of between 6 and 8 per 100,000 in most Western populations (1). Surgical clipping of intracranial aneurysms is increasingly being replaced by endovascular treatment with coils. The recent publication of a multicenter randomized trial showing improved safety and clinical outcome of patients treated with endovascular methods as compared with open clipping has accelerated this trend (2). The results of this trial have increased the numbers of patients referred for endovascular treatment and thereby further emphasized the need for development of new techniques and devices to enhance the ability to treat intracranial aneurysms effectively.
Wide-necked aneurysms are difficult to treat, both surgically and endovascularly, because of their unfavorable geometry, which reduces the possibility of achieving dens packing and elimination of aneurysm from circulation. Endovascular strategies for managing wide-necked aneurysms have previously included the balloon remodeling method described by Moret et al (3) and the use of 3D coils (4, 5). Other than the classical role in revascularization procedures, stents started to be used more frequently as a support to keep the coils inside wide-necked aneurysms and allow dense packing of the aneurysm lumen.
After validation of the technique by experimental studies (6, 7) reports of stent-assisted embolization for the treatment of wide-necked aneurysms in human appeared in literature (8–11). Most of them are from coronary practice and balloon expandable. In addition to the pressure-driven deployment method, their profile and stiffness increase the risk of vessel damage. Navigational difficulty in the tortuous cerebral circulation is another technical limiting factor for these devices. In a substantial number of cases, the need for permanent support for the coils to protect the parent artery is still obvious. Until recently, no stent that is optimized for intracranial use was available.
We report the result of our experience in by using Neuroform stent for the treatment of wide-necked cerebral aneurysms. Technical success, efficacy, and complications related with the method were assessed.
Methods
A retrospective review of all patients treated with Neuroform stent-assisted coiling (SAC) during the interval between September 2002 and December 2003 was done. Information was gathered retrospectively from an internal endovascular aneurysm treatment database, patient medical records, and image archive system (PACS). Informed consent from the patients and institutional review board approval for the humanitarian use of Neuroform device and retrospective review of the data were obtained. Thirty-five aneurysms in 32 patients (7 male and 25 female) were treated with SAC (Table 1). The mean age of the patients was 58.6 years (range, 29–82 years). Patients having intracranial aneurysm (ruptured or unruptured) with a dome-to-neck ratio <2 or neck size >5 mm as measured from a pretreatment 3D rotational digital-subtraction angiography (3D-DSA) were included. Stent deployment was attempted in 35 aneurysms. Four were ruptured—two of which were acute—and five were recurrent aneurysms, which had been previously treated by endovascular and/or surgical methods; the others were unruptured with no previous attempt of treatment. Mean dome size, mean neck size, and mean dome-to-neck ratio were 9.1 mm, 6.5 mm, and 1.50, respectively. Other than one fusiform aneurysm, all aneurysms were wide-necked saccular aneurysms, seven of which were located in the posterior circulation.
Summary of patients treated with neuroform assisted coilinga
Preoperative Evaluation and Medication
In addition to standard multiprojection cerebral angiography, 3D-DSA of the vessel bearing the aneurysm was acquired in all patients. Morphologic evaluation and measurements of the parent vessel and aneurysm were done on a separate workstation by using reconstructed 3D-DSA images. Patients having unruptured aneurysm were premedicated for 3 days before treatment with 300 mg acetyl salicylic acid (ASA) and 75 mg clopidogrel. No patient with ruptured aneurysm was pretreated with antiplatelet drugs.
Neuroform Stent and Delivery System
The Neuroform stent is a self-expanding nitinol (nickel-titanium alloy) stent with a thermal memory (12). It has an ultra-thin, highly porous design with low radial force. For delivery, the stent is preloaded into a 3F braided microcatheter. During delivery, a 2F microcatheter fills the annular space in the 3F catheter and is used for support during retraction of the delivery catheter (unsheathing) for stent deployment. Four platinum markers on each end of the stent are the only elements that are visible fluoroscopically.
SAC Technique
All procedures were performed under general anesthesia. After completion of the diagnostic angiogram, all patients were anticoagulated with heparin adequate to keep the activated clotting time longer than 2–2.5 times the baseline value. A 6- or 7F guiding catheter was placed within the appropriate internal carotid or vertebral artery. In our early experience, a standard microcatheter and flexible guidewire were used to cross the segment of artery from which the aneurysm arose. The standard wire was then exchanged for an exchange length wire over which the Neuroform stent was delivered and deployed. Later, the Neuroform was delivered directly across the aneurysm neck by using a standard flexible tip guidewire placed through the 3F catheter containing the stent (Fig 1). Keeping at least a 4-mm margin between the ends of the stent and aneurysm neck and at least 0.5 mm over sizing the stent diameter to the diameter of the artery into which it is to be deployed is advocated for good anchoring and stability of the stent.
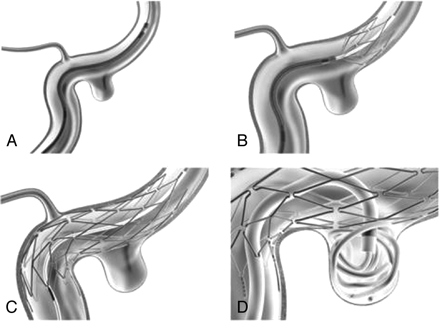
Schematic illustration of Neuroform-assisted embolization of a wide-necked superior hypophyseal aneurysm.
A, Microcatheter containing the stent was positioned over the wire distal to aneurysm location.
B, The stent was deployed by holding the stabilizing catheter in a fixed position while the 3F catheter was pulled back.
C and D, Interstices of fully expanded stent can easily accommodate microcatheter for coiling. Protrusion of coil loops in to internal carotid artery is prevented by the stent.
In 14 patients, SAC was performed in two separate sessions. Time intervals between placement of the stent and coiling were between 1 and 148 days (mean, 44 days). The remaining 18 aneurysms were embolized immediately after stent deployment. In 11 patients, because of the inability to achieve a working projection adequate to ensure that coils were not herniating through the stent, balloon neck protection was also used.
After stent deployment, the aneurysms were catheterized using a variety of standard microcatheters and guidewires. The aneurysms were then coiled by using standard techniques with a variety of commercially available coils.
In most instances, homeostasis was achieved by use of an arterial closure device and heparin was not reversed at the end of the procedure. Patient with unruptured aneurysm maintained on the same antiplatelet regimen for a month, followed by lifelong ASA if otherwise indicated by different medical condition of the patient. In patients with ruptured aneurysms, the same postprocedure antiplatelet regimen (300 mg ASA + 75 mg Clopidogrel) was initiated immediately after the embolization through the Nasogastric tube. All patients were scheduled for 6-month clinical follow-up and angiographic evaluation.
Observations
The technical success of the procedure, procedure-related complications, angiographic results, and neurologic outcome were recorded. Angiographic results were classified according to the classification used by Raymond and Roy (13) with slight modification, such as complete occlusion without neck remnant, subtotal but satisfactory occlusion (>90% with small neck remnant or dog ear), residual filling of the aneurysm lumen, and failure.
Results
Stent Placement and Embolization
In 30 of the 34 aneurysm-bearing vessel segments, the stent was successfully delivered and deployed at the first attempt. Three of the remaining four were stented successfully on a second attempt and, in one instance, the stent could not be deployed. Technical success of the Neuroform stent deployment to the desired location was 97%. Coil embolization was attempted in 33 of 34 stented aneurysms. In one patient, coiling of an unruptured carotid ophthalmic aneurysm that had previously been stented was postponed because of complications that occurred during coiling of a contralateral aneurysm (patient 11).
Thirty of 33 attempts at coiling were successful (91%). In two instances, coiling was not successful because of coil protrusion. In a patient with right superior hypophyseal internal carotid aneurysm with dome and neck size of 4 mm (patient 8) multiple attempt of placing the first coil safely within the aneurysm lumen was not successful (Fig 2). In the case of patient 10, we cancelled the coiling after stent placement within the parent vessel. In that case, an 11-mm right vertebral artery aneurysm was located at the origin posterior inferior cerebellar artery (PICA). Although the Neuroform stent, placed at vertebral artery, covered the 8-mm neck and protected the vertebral artery from coil protrusion, we observed diminished flow at the right PICA before detachment of the first coil. Because of anticipated high risk of PICA stroke we retracted the coil and stopped the procedure (Fig 3). The third case that we failed to embolize had a left ophthalmic aneurysm (patient 11). We tried balloon-neck protection during embolization, but, unfortunately, aneurysmal or vessel rupture occurred during the initial balloon inflation. As a result, parent artery occlusion was performed. Immediate posttreatment angiography revealed complete aneurysm occlusion in 5 cases, partial but satisfactory occlusion in 15, and residual filling in 10. One aneurysm with residual filling was retreated and partial, but satisfactory occlusion was achieved (patient 5). Posttreatment angiographic follow-up was available in 12 patients (range, 2–14 months). In nine, the degree of aneurysm occlusion was either stable or had improved. In the remaining three, minor recanalization was detected without significant increase in size of the aneurysm. Retreatment was performed in only one of them. No SAH vessel occlusion, stenosis at the stented vessel segments or late stroke at the treated vessel territory was documented after the treatment during the follow-up period.
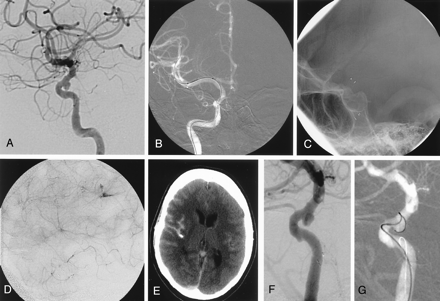
Case 8.
A, Pretreatment lateral carotid angiography shows a broad-necked superior hypophyseal aneurysm of the right internal carotid artery.
B, Stent delivery system is advanced distal to the aneurysm over microguidewire. Distal marker of the stent delivery catheter, proximal and distal markers of the stent itself within the catheter are visible.
C, Lateral fluoroscopic view shows the stent in the cavernous internal carotid artery, covering the orifice of the aneurysm.
D, Late arterial phase of lateral carotid angiography obtained after the stent deployment shows contrast extravasation, which is confirmed by CT (E) also. F and G, The second session of embolization performed 3 weeks later failed because of the persistent protrusion of the coils into the internal carotid artery.
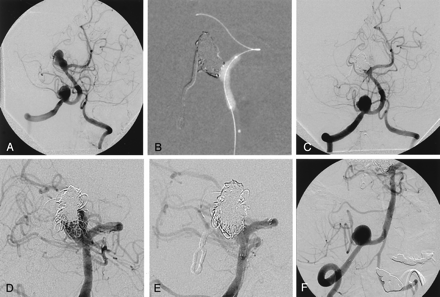
Case 10.
A, Left vertebral angiography obtained 6 months after previous surgery shows residual filling of the basilar tip aneurysm and additional right vertebral artery aneurysm close to the origin of the posterior inferior cerebellar artery. Right posteriocerebral artery is supplied by the right carotid circulation via right posterior communicating artery (not shown).
B, Fluoroscopic road map image during embolization with balloon remodeling technique and postembolization right vertebral angiogram (C) shows satisfactory occlusion of the aneurysm with small neck remnant, especially on the right side.
D, Follow-up angiography 22 months after the embolization reveals recanalization and regrowth of the aneurysm.
E, After placement of Neuroform stent extending from the left posterior communicating artery to the distal basilar artery recanalized portion of the aneurysm embolized with multiple coils.
F, Proximal and distal markers of second Neuroform stent, placed across the right vertebral artery aneurysm, are seen. After insertion of the first coil into the aneurysm, lumen flow within the right posterior inferior cerebellar artery is diminished. The coil is retracted and embolization is abandoned.
Complications and Adverse Events
Procedure related mortality occurred in one patient (3.1%). This occurred after treatment of a large, ruptured, wide-necked basilar apex aneurysm that had recurred despite previous treatment on two occasions with dual balloon-neck-protection technique (Table 2). Two Neuroform stents were placed so that one passed through the other forming a Y at the junction between the two posterior cerebral arteries and the aneurysm (patient 28). A satisfactory result with a small residual neck immediately after treatment was achieved, and the patient was discharged with no adverse events associated with the procedure. Two weeks after discharge and after discontinuing his antiplatelet medications, he suffered a basilar artery thrombosis resulting in multiple brain stem and cerebellar infarcts. He died shortly thereafter.
Complications and adverse events
In eight of the SAC procedures, adverse events occurred (25%). In one case, the aneurysm ruptured at the time of coiling during initial balloon inflation. Parent artery occlusion was performed (patient 11) and a right hemiplegia and aphasia occurred. At the 6-month follow-up examination, her motor deficit was almost completely resolved but a motor aphasia persisted. In another patient, after a successful stent deployment minimal extravasation of contrast medium was noted in the right posterior parietal region, adjacent to posterior parietal branch of the right middle cerebral artery most probably due to vessel injury with the microguidewire used for stent deployment (patient 8; Fig 2). Systemic anticoagulation was immediately reversed with protamine, and the procedure was terminated. The patient awoke from general anesthesia with a complaint of headache but no change from her baseline neurologic status. Cranial CT revealed an SAH. She recovered fully and had no deficit at the time of discharge. In a patient having fusiform right posterior cerebral artery aneurysm (patient 25), we tried SAC to embolize the aneurysm without parent vessel occlusion, but unfortunately the deployed Neuroform stent failed to supply necessary support to reconstruct the vessel. Diseased segment of posterior cerebral artery was occluded. Although small ipsilateral occipital infarct had developed the patient was asymptomatic without any visual field defects. One patient with a right paraophthalmic aneurysm lost vision in the ipsilateral eye immediately after coiling (patient 9). Her vision was limited only to light perception at the 3-month follow-up. Another patient with a paraophthalmic aneurysm (patient 31) experienced recurrent, transient retinal ischemia 2 weeks after the procedure. One patient experienced an ipsilateral putaminal hemorrhage 9 days after the SAC procedure (patient 14). Groin hematoma necessitating surgical repair and severe contrast reaction were other procedure-related adverse events.
Apart from these clinically manifested complications, thrombus formation was noted at immediate poststent placement angiography in six cases. These were seen as 1–2-mm fuzzy irregular filling defects that are attached to stent struts (Fig 4). All of these patients were treated with a platelet glycoprotein IIb/IIIa inhibitor (abciximab, ReoPro, Lilly, Indianapolis, IN) given either intraarterially or intravenously. Complete resolution of the thrombus was seen in four cases after intravenous bolus. One thrombus, still present after administration of the intravenous bolus was not observed on angiogram performed next day (Fig 4). In the other case, a very small residual thrombus persisted on the stent surface at the time of the 24-hour control angiogram. None of these six patients developed neurologic symptoms, although in one case significant groin hematoma occurred after the removal of the arterial sheath 24 hours after treatment.
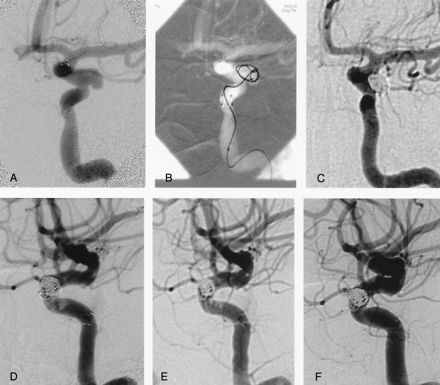
Case 3.
A, Left carotid angiogram obtained after deployment of Neuroform stent shows broad-necked aneurysm originating from the lateral wall of the internal carotid artery at the level of ophthalmic artery.
B, Advancement of the first coil in to the aneurysm through interstices of the stent.
C, Total occlusion of the aneurysm is seen on postembolization anteroposterior left carotid angiography.
D, On lateral view, ill-defined filling defects consistent with significant amount of fresh thrombus is seen within the stented segment of the left internal carotid artery proximal to aneurysm. Partial and complete lysis of the clot is seen on angiograms obtained 15 minutes (E) and 24 hours (F) after starting the intravenous abciximab protocol.
Discussion
After 1995, when the Guglielmi detachable coil system (Boston Scientific/Target, Fremont, CA) received U.S. Food and Drug Administration (FDA) approval, criteria for endovascular treatment broadened. Today, only aneurysms that, for anatomic or other reasons, seem unsuitable for coil occlusion are excluded from endovascular therapy. Wide-necked aneurysms are the most frequent treatment challenges for endovascular surgeons because of the risk of coil-loop herniation through the broad neck into the parent artery. Despite significant advances in coil design (e.g., two and and 3D coils) as well as the development of modified techniques of coiling (e.g., balloon neck protection) there remains a substantial number of aneurysms that either cannot be treated successfully with coiling or that, when treated, recur at a high incidence (3–5). In the past decade, several reports describing the experimental and clinical use of peripheral or coronary stents for stent-assisted embolization have appeared in the literature (8–13). One of the largest series by using stents not specifically designed for use in the cerebral vasculature was reported by Lylyk et al (14). They reported cerebrovascular stent placement in 111 patients having either occlusive atherosclerotic disease or aneurysms; in their series, the overall mortality and morbidity were 6.3 and 10.9%, respectively. In 62 of these 111 patients, stent placement was used before coiling of intracranial aneurysms. The overall success in deploying the stent at the appropriate site was 90.3%. In 19% of these, suboptimal placement such that it was necessary to deploy a second or third stent was noted.
Fundamental design characteristics of balloon mounted coronary stents have significantly limited their use in intracranial aneurysm therapy. They do not have enough flexibility for the navigation of tortuousities inherent to cerebral circulation. Rigid structure precludes their use at curved vessel segment such as carotid siphon and excessive force exerted during deployment may also cause injuries to the vessel wall.
An ideal stent for SAC should have a low profile, be flexible, and be made of material that is self-expandable so as to accommodate the complex geometry of intracranial arteries. Also, the stent strut design and concentric radial force of the device is important, because this feature supplies the support necessary to prevent coils from herniating into the parent artery. To the best of our knowledge, the first FDA-approved stent, designed according to these criteria, is the Neuroform stent (Boston Scientific/Target). This device is a self-expanding nickel-titanium alloy stent with an ultra-thin, open-cell mesh design. It has a high degree of elasticity and thermal memory with a radial force adequate to constrain coils within an aneurysm. It is available in a variety of diameters and lengths, ranging from 2.5 to 4.5 mm and 10 to 20 mm, respectively. Because of the ultra-thin struts, it is essentially radiolucent and is only visible fluoroscopically because of four radiopaque platinum marker bands placed at each of its ends. A 2F stabilizer catheter is used as an over-the-wire pusher for deployment of the stent with an unsheathing maneuver. The low profile and relatively smooth surface of the delivery system achieved by positioning the stent in the 3F microcatheter facilitate navigation through tortuous courses. In our experience, deployment of the stent has sometimes been impossible when the 2F stabilizer catheter is used. Because of this, we often have deployed the stent by using either a coil pusher or a 0.018-inch guidewire.
In a recent series including 19 patients treated with Neuroform-assisted embolization significant difficulty was encountered while attempting to deploy the stent. In two of them the stabilizer and microwire were removed and the stents were extruded by other means (0.016-inch coil pusher). As a result, suboptimal stent positioning after deployment was reported in three instances (15). We did not experience any inaccurate deployment, but in one patient we were unable to navigate the stent to the desired position. This aneurysm was coiled subsequently by using only a balloon remodeling technique. Although the stent’s radial force is less than that of stents designed for use in atherosclerotic disease, it was, in general, sufficient to confine coils within the aneurysm lumen. In one case, although the neck of the aneurysm was covered with stent, protrusion of the first coil could not be prevented (Fig 2). In this particular case, aneurysm dome and neck size were both 4 mm and unfavorable orientation of the stent struts across the neck may result in insufficient support for small coils. In our series there are five more aneurysms that are similar in size (dome size 5 mm or less). In three of them, balloon protection was found to be necessary. We believe the size of the aneurysm and the first coil play a major role. One should be aware that risk of coil protrusion through the stent would be higher if the first coil size is <4 mm.
The combination of stent placement with balloon remodeling has been described elsewhere (16). Basically, there are two main indications for additional use of balloon. Neuroform stent may sometime be insufficient to confine coils in an aneurysm because of open cell structure and the low radial force. Low visibility of the stent struts and inability to achieve a working projection adequate to ensure that coils were not herniating through the stent also make the balloon protection necessary. In 11 of our patients, we attempted to employ this technique during coil placement. A soft, compliant balloon was used in all cases (Hyperglide, Micro Therapeutics, Irvine, CA). In one instance, navigation of the balloon across the aneurysm neck could not be achieved. In one case, during the first inflation of the balloon, the aneurysm or vessel ruptured but the direct relationship between the presence of stent and rupture could not be defined. No similar event had occurred in remaining nine cases in which the stent balloon remodeling technique were applied.
Adverse events in our series can be grouped in two categories: thrombotic ischemic and hemorrhagic. An ipsilateral putaminal hemorrhage (9 days after the SAC), a groin hematoma, and vessel rupture leading to SAH in two instances constitute hemorrhagic complications. Although the putaminal hemorrhage occurred in the subacute phase and was not directly related to the procedure, posttreatment dual antiplatelet therapy might have been a precipitating factor.
Four patients suffered neurologic deficits because of procedure-related thromboembolic events. In two of these cases, ocular ischemic symptoms occurred, leading to loss of vision in one eye and a retinal transient ischemic attack in the other patient. Propagating thrombus from the aneurysm into the ophthalmic artery likely explains these events. The single mortality in our series resulted from a late subacute thromboembolic complication occurred in a patient with recurrent basilar tip aneurysm treated with two Neuroform stents, One patient with a fusiform right posterior cerebral artery aneurysm developed a small occipital infarct after parent artery and aneurysm occlusion.
Thrombogenicity of the stent and precautions by itself to overcome this problem such as anticoagulation and pre- and postoperative antiplatelet therapy are main causative factors for increased risk of stent-assisted embolization. Therefore, adjustment of coagulation status of the patient during and after the procedure plays a major role. There are controversial reports about benefit of the dual antiplatelet therapy (17, 18). In our practice, dual antiplatelet therapy (ASA and clopidogrel) is given for 3–7 days before the treatment of unruptured aneurysms. For acutely ruptured aneurysms, antiplatelet treatment is not instituted until the aneurysm is coiled. Our posttreatment regimens generally consist of 4-week continuation of the dual therapy and then lifelong ASA, unless this is contraindicated. During the procedure, anticoagulation is maintained by heparin infusion aiming for elevation of the ACT to 2–2, 5 times the baseline value. We believe antiplatelet and anticoagulation therapy should be tailored according to the results of ongoing researches.
Preliminary experiences reported in the literature indicate that glycoprotein IIB/IIIA inhibitors such as abciximab are useful agents for treatment of thrombotic complication during neuroendovascular interventions. Although several case series were reported with different regimens, we believe more extensive clinical experience is needed to validate the safe use of glycoprotein IIB/IIIA inhibitors and to define optimal dose and delivery method (19, 20). Currently we use a low-dose intravenous regimen (30 μg/kg intravenous bolus followed by 10 μg/kg/h infusion for 12 hours). In two cases, bolus doses were given intraarterially through the microcatheter. No intracranial hemorrhagic complication related to this regimen was observed. Prophylactic use of abciximab has been reported (14) for stent placement of complex intracranial atherosclerotic lesions, but we believe it is not justified for SAC procedures.
In our series, the overall rates of adverse events and procedure-related morbidity causing permanent neurologic deficit were 25% and 9.3%, respectively. Four incidents of thromboembolic complication (two of them subclinical), six groin or retroperitoneal hematomas (four of them required transfusion), and one hypertensive hemorrhage were reported by Fiorella et al (15). Two procedure-related deaths (10.5%) occurred in their patient group.
Although the rate of complication resulting permanent neurologic deficit was reported to be between 0% and 4.5% with balloon-assisted embolization for wide-necked aneurysms, the results of large cohorts revealed higher morbidity (7%) for endovascular treatment, even for unruptured aneurysm without unfavorable morphologic selection (21–23). As expected, complication rates for the ruptured aneurysms are much higher; 23.7% of patients allocated to endovascular treatment were reported as dependant or dead at the first-year follow-up of the International Subarachnoid Aneurysm Trial (2). Our rate of complication resulting in permanent neurologic deficit was comparable to the results of unruptured aneurysm cohort. Most of our complicated cases were treated during the first half of our experience period with Neuroform. We believe the SAC procedure will be safer after a learning-curve period.
Stent placement and coiling was performed in two stages in 14 cases. Although we have not experienced any inadvertent movement of a stent during catheterization of the aneurysms when the procedure is done at a single sitting, we believe that, in some instances, a staged treatment offers some advantages. These include shortening of the procedure, lower contrast medium load, and the healing of any minor endothelial injury that might have occurred during stent positioning and deployment. A similar approach was used in one-third of the patients treated with SAC reported by Lylyk et al (14).
Conclusion
The Neuroform stent is a new endovascular tool for the treatment of wide-necked aneurysms not amenable to coil embolization alone. Delivery of the stent to the desired location is easy and accurate, but we experienced deployment difficulties with 2F stabilizer catheter of both first- and second-generation stent delivery systems. Because of its thin filaments and highly porous design, no adverse effect on parent vessel such as intimal hyperplasia, stenosis, or occlusion was observed, but longer follow-up examinations are required to support early observations. Well-adjusted antiplatelet and anticoagulation treatment is crucial because of thrombogenic properties inherent to the stent. Although the need for several technical improvements in the Neuroform stent is evident, it offers a therapeutic alternative in the treatment of wide-necked and complex aneurysms.
Footnotes
Presented at the 42nd annual meeting of the American Society of Neuroradiology, Seattle, WA, June 7–11, 2004.
S.A. is sponsored for research studies by Turkish Council of Scientific and Technical research. C.M.S. and M.E.M. are scientific consultants for Boston Scientific.
References
- Received July 7, 2004.
- Accepted after revision November 6, 2004.
- Copyright © American Society of Neuroradiology