Abstract
PURPOSE: To evaluate the utility of perfusion CT (PCT) combined with CT angiography (CTA) for the diagnosis and management of vasospasm, by using conventional digital subtraction angiography (DSA) as the gold standard.
METHODS: We retrospectively identified 27 patients with acute subarachnoid hemorrhage who had undergone CTA/PCT, DSA, and transcranial Doppler (TCD) ultrasonography within a time interval of 12 hours of one another. The patients’ charts were reviewed for treatment of vasospasm. CTA, PCT, TCD, and DSA examinations were independently reviewed and quantified for vasospasm. PCT thresholds, CTA findings, noncontrast CT (NCT) hypodensities, and TCD thresholds were evaluated for accuracy, sensitivity, and specificity, as well as for negative (NPV) and positive predictive values (PPV) in the prediction of angiographic vasospasm and endovascular treatment, considering DSA as the gold standard.
RESULTS: Thirty-five CTA/PCT, TCD, and DSA examinations were performed on these 27 patients. A total of 123 arterial territories in 11 patients demonstrated angiographic vasospasm. Six patients underwent endovascular therapy. CTA qualitative assessment and PCT-derived mean transit time (MTT) with a threshold at 6.4 seconds represented the most accurate (93%) combination for the diagnosis of vasospasm, whereas MTT considered alone represented the most sensitive parameter (NPV, 98.7%). A cortical regional cerebral blood flow value ≤39.3 (mL × 100 g−1× min−1) represented the most accurate (94.8%) indicator for endovascular therapy. PCT had significantly higher PPV (89.9%) than TCD (62.9%).
CONCLUSIONS: A CT survey combining CTA and PCT represents an accurate screening test in patients with suspected vasospasm. However promising, the value of PCT for selecting the best management strategy in such patients will need to be further investigated.
Aneurysmal subarachnoid hemorrhage (SAH) is associated with as much as 67% patient fatality and 10%–20% long-term dependence in survivors.1, 2 Vasospasm represents the leading cause of death and disability in patients who survive the initial event.1 The prevalence of vasospasm during the first 2 weeks following SAH is as high as 70%, and 50% of the patients with angiographic vasospasm will eventually develop ischemic neurologic deficits.3 Early recognition and prompt treatment of vasospasm can improve neurologic outcomes.4–6
The best clinical indicator of significantly reduced brain perfusion due to vasospasm is the presence of new neurologic deficits. Clinical deficits, however, are often nonspecific. In particular, the detection of such findings may be delayed in patients who have significant neurologic impairment at baseline or who require sedation during intensive care management.7
Current methods for diagnosing vasospasm include transcranial Doppler (TCD) ultrasonography and the current gold standard of digital subtraction angiography (DSA). The major advantage of DSA lies in its accuracy and the capacity to immediately perform endovascular treatment by balloon angioplasty and/or intra-arterial injection of vasodilatory drugs.8–10 DSA is an invasive technique, however, that may require general anesthesia and is associated with a slight risk of procedure-related stroke. The diagnosis of anatomic vasospasm is straightforward, but DSA is limited in its ability to quantify cerebral blood flow or the risk of cerebral ischemia leading to stroke.
TCD is commonly used to detect vasospasm noninvasively and determine which patients should be further evaluated by DSA. Nevertheless, TCD has several pitfalls. It can identify vasospasm with only 67% specificity compared with DSA,11 with false-positive readings occurring when patients are treated with medications that increase blood pressure.12 TCD sensitivity and specificity can also vary greatly, depending on the level of experience of the operator, and only 85% of patients will have temporal bone windows that allow reliable TCD measurements.13 Finally, increases in blood flow velocities (FVs) measured by TCD14 do not correlate with brain perfusion values and cannot reliably distinguish between patients with vasospasm of various severity.15
Because TCD is not completely accurate, a noninvasive imaging technique affording more accurate assessment and quantification of vasospasm is desirable. The ideal technique would accurately detect the presence or absence of vasospasm before the occurrence of clinically apparent deficits and would also distinguish the severity of the disease, to stratify treatment. For this application, CT angiography (CTA) and perfusion CT (PCT) techniques have significant advantages over TCD.
CTA is currently being used in the setting of acute stroke to accurately assess vessel patency and facilitate early selection of patients for aggressive stroke treatment.16 CTA is also used for screening of intracranial aneurysms and in selective cases allowing for aneurysm surgical treatment without DSA.16,17 CTA is noninvasive and can be performed immediately after conventional CT, a major logistic advantage in the emergency setting, when rapid treatment decisions are necessary. In addition, patients who are critically ill or uncooperative can undergo CTA evaluation with minimal sedation because of the rapid acquisition of CT data. CTA has been reported to have 64% sensitivity and 96% specificity in the assessment of the location and severity of vasospasm after SAH.18,19
Similarly, the PCT technique, which assesses brain perfusion hemodynamics, is noninvasive, can be performed repeatedly, and can easily be incorporated into the standard CT/CTA protocol that is classically performed for SAH patients with suspected vasospasm. PCT uses standard CT equipment and requires only dedicated postprocessing software that can generate perfusion maps of the brain within 5 minutes of data acquisition. Unlike TCD, PCT can assess blood flow throughout the whole territory perfused by a cerebral artery. Quantitative PCT maps have been validated by comparison with xenon CT20 and positron-emission tomography (PET) studies.21 PCT has a role in the early management of adult patients with acute stroke and of other cerebrovascular disorders22–24 because it affords insight into the relative areas of cerebral infarction and the associated ischemic penumbra. Experience with PCT in vasospasm following SAH, however, is limited. To date, only one small series has been reported,25 and there has been no systematic comparison with DSA or TCD results.
The goal of this study was to evaluate the utility of PCT combined with CTA for the diagnosis and management of vasospasm, by using DSA as the gold standard.
Methods
Design
At our institution, all patients with an acute aneurysmal SAH are monitored for vasospasm with serial neurologic examinations and daily TCD studies. When the clinical findings are suspicious or TCD FVs are elevated, conventional DSA is performed. In some cases, CTA and PCT are performed before DSA. Angiographic vasospasm is treated with intra-arterial verapamil or endovascular balloon angioplasty when appropriate. In addition, standard medical treatment for SAH patients includes oral nimodipine26 and hypertensive therapy, with the goal of increasing the mean arterial blood pressure to 100–120 mmHg.27, 28 External ventricular drainage is used to treat symptomatic hydrocephalus.
Between January and September 2003, all patients with aneurysmal SAH who underwent DSA because of a suspicion of vasospasm were retrospectively identified. Patients were included in the present study if they underwent CTA/PCT and DSA (with or without endovascular treatment) within a 12-hour interval. The TCD examinations performed the same day as the CTA/PCT were also included in the analysis.
Our institutional review board approved this retrospective review of patient studies obtained as part of standard clinical care.
Imaging Protocol and Data Processing
The CT protocol for patients with suspected vasospasm includes a noncontrast CT (NCT) of the brain, CTA, 2-level PCT series, and contrast-enhanced CT of the brain.
The CTA data acquisition was performed according to the following protocol: spiral mode, 0.6-second sections; collimation, 8 or 16 × 1.25 mm; pitch, 1.75; section thickness, 1.25 mm; reconstruction interval, 1.00 mm; acquisition parameters, 120 kVp/240 mA. A caudocranial scanning direction was selected, covering the whole brain down to 1 cm below the foramen magnum, to encompass the posteroinferior cerebellar arteries in the volume analysis. Seventy milliliters of iohexol (Omnipaque, Amersham Health, Princeton, NJ; 300 mg/mL of iodine) were administered into an antecubital vein by using a power injector at an injection rate of 4 mL/s. Adequate timing of the CTA acquisition was achieved according to a bolus-chasing technique. CTA raw data were reformatted in axial, sagittal, and coronal 3-mm-thick maximal intensity projection (MIP) images.
PCT was performed 5 minutes after the CTA. PCT data acquisition consisted of a 45-second series, with 45 gantry rotations performed in cine mode during intravenous administration of iodinated contrast material. The temporal sampling rate was one rotation of the gantry per second on acquisition. Multidetector raw CT technology permitted the assessment of 2 adjacent 10-mm-thick sections for each series. Two 10-mm-thick sections were preferred to 4 adjacent 5-mm-thick sections per location, to maximize signal intensity-to-noise ratio for the same acquisition parameters. Two successive PCT data acquisitions were performed, separated by 3–5 minutes. The 2 sections of the first PCT level were at the level of the third ventricle and the basal ganglia, positioned above the orbits to protect the lenses. The second PCT series was selected at a level of 3.5 cm rostral to the first section of the first series. For each series of PCT, a bolus of 40 mL of iohexol was administered into an antecubital vein (right arm preferred) by using a power injector at an injection rate of 4 mL per second. The acquisition parameters for both PCT series included 80 kVp and 120 mAs. CT scanning was initiated 7 seconds after initiation of contrast bolus injection. The delay before contrast material reached the brain parenchyma allowed the acquisition of baseline images without contrast enhancement.
TCD FVs were recorded with a Neuroguard ultrasonographic system (Medasonics, Fremont, Calif) in the internal carotid artery bifurcations (ICABs), anterior cerebral (ACA), middle cerebral (MCA), posterior cerebral (PCA), vertebral (VA) and basilar (BA) arteries, as well as in the external carotid arteries (ECA). In addition to the absolute FV values, extracranial-to-intracranial (EICA) ratios were calculated as the ratio between the FV in one specific artery and the FV in the ECA.29, 30 The concept of EICA ratio does not apply to the PCA, because it is most often supplied by the vertebrobasilar system. Instead, a BA-to-VA ratio was calculated according to the following formula: BA FV/[(right VA FV + left VA FV)/2].
Three- or 4-vessel DSA was performed via a transfemoral approach under monitored sedation or general anesthesia. Conventional angiographic views (frontal, lateral, oblique) were obtained, as were dedicated magnified and focused views on the treated aneurysmal sites.
Data Analysis
The NCT examinations were reviewed by an experienced neuroradiologist for the presence or absence of hypoattenuation.
The PCT data were analyzed by using software developed by Philips Medical Systems (Cleveland, Ohio). This software relies on the central volume principle, which is the most accurate for low injection rates of iodinated contrast material.31 The central volume principle uses mathematical deconvolution to calculate the mean transit time (MTT).32 The regional cerebral blood volume (rCBV) map is calculated from the areas under the time-enhancement curves.33 Finally, a simple equation combining rCBV and MTT values allows the calculation of regional cerebral blood flow (rCBF): rCBF = rCBV/MTT.15–17 Time-to-peak (TTP) values were also calculated. rCBV, MTT, rCBF, and TTP values were recorded in the gray matter of the arterial territories listed in Table 1.
Arterial territories assessed by the different imaging techniques
CTA MIP images were reviewed by a neuroradiologist for vasospasm, which was qualified as absent (0), present and mild/moderate (1), or present and severe (2). When the reviewer could not decide between a hypoplastic and a vasospastic segment of the circle of Willis, the considered segment was qualified as “indeterminate” and considered as negative with respect to vasospasm. The reviewer also evaluated the quality of the CTA images (metallic artifacts, quality of the contrast material bolus).
All TCD examinations were performed by the same technologist. The following thresholds were used in the interpretation of absolute values and EICA ratios11, 13:
For the vertebrobasilar system, the following thresholds were used11, 13:
The DSA examinations were performed and interpreted by an interventional neuroradiologist for vasospasm. Vasospasm was graded as absent (0), present but not requiring endovascular treatment (1), or present and requiring endovascular treatment (2).
Because the goal of the present study was to compare CTA/PCT, TCD, and DSA techniques for the evaluation of vasospasm, we developed and used the anatomic chart reported in Table 1 to compare the values in the different arterial territories as assessed by the different imaging techniques. For each patient, 18 arterial territories were assessed and compared across techniques.
Statistical Analysis
The patients and assessed arterial territories were distributed into the following groups:
1 + 2. No angiographic vasospasm
3. Angiographic vasospasm but no endovascular treatment,
4. Angiographic vasospasm and endovascular treatment.
To compare PCT and TCD, the group of patients with no vasospasm (1 + 2) diagnosed on DSA was further subdivided into 2 subgroups:
No vasospasm diagnosed on DSA or on TCD
Vasospasm diagnosed on TCD but not on DSA (TCD false-positives), leading to a final 4-group distribution.
PCT values were evaluated for statistically significant differences between the 4 groups by using Kruskall-Wallis tests. When the Kruskal-Wallis test was positive and significant (P < .001), the statistical significance was checked by comparing each pair of groups by using Wilcoxon (Mann-Whitney) tests. For both the Kruskal-Wallis and the Wilcoxon tests, the significance level was set at 0.001 to account for the multiple testing (Bonferroni).
For each PCT parameter, a “diagnosis” threshold was calculated as the mid-value between the average PCT values in the group of patients with no angiographic vasospasm and in the group of patients with angiographic vasospasm (with or without endovascular treatment). Similarly, a “treatment” threshold was calculated as the mid-value between the average PCT values in the group of patients with angiographic vasospasm but no endovascular treatment and in the group of patients with angiographic vasospasm and endovascular treatment.
The PCT “diagnosis” thresholds for each parameter were evaluated for accuracy, sensitivity, and specificity, as well as negative (NPVs) and positive predictive values (PPVs) in the prediction of territories with angiographic vasospasm. As a reminder, the accuracy of a test designates the number of true results (true positives + true negatives) divided by the total number of subjects. Selected combinations of PCT parameters and CTA were also considered. Similarly, the accuracy, sensitivity, specificity, NPV, and PPV of NCT hypodensities, CTA findings, and TCD-absolute FV (120 cm/s), and EICA ratio thresholds (3.00) were also assessed for the diagnosis of present or absent angiographic vasospasm.
The PCT “treatment” thresholds for each parameter, but also CTA (severe vasospasm) and TCD absolute FV (180 cm/s) and EICA ratio thresholds (6.00), were evaluated for accuracy, sensitivity, specificity, NPV, and PPV in predicting whether an endovascular treatment was performed or not during DSA.
Results
Patients
Between January and September 2003, 112 patients were admitted to our institution with aneurysmal SAHs. A total of 27 patients with CTA/PCT and DSA within 12 hours were identified. Seven were men and 20 were women, with a median age of 56 years (range, 18–82 years). The presenting clinical World Federation of Neurosurgical Societies grades were as follows: grade 1, 2 patients; grade 2, 9 patients; grade 3, 8 patients; grade 4, 5 patients; grade 5, 3 patients. A total of 29 aneurysms were identified in these patients (3 ICABs, one superior hypophyseal artery, one carotid ophthalmic artery, 8 anterior communicating arteries [AComs], 4 MCA bifurcations, 10 posterior communicating arteries [PComs], one BA tip, one anterior inferior cerebellar artery), including 27 ruptured aneurysms, treated either by neurosurgical clipping18 or endovascular coiling.9
Seven patients were found to have no vasospasm on both DSA and TCD. Nine patients were suspected of vasospasm on TCD but turned out to be vasospasm-negative on DSA. Eleven patients were found to have angiographic vasospasm. Six of these 11 patients underwent endovascular therapy with intra-arterial verapamil and/or balloon angioplasty. It is interesting to note that, among the patients with posterior circulation aneurysms, the patient with the right anterior inferior cerebellar artery did not show vasospasm; the patient with the basilar tip aneurysm demonstrated vasospasm involving bilateral PCAs and was treated with intra-arterial verapamil.
Imaging Examinations
Because of a clinical suspicion of vasospasm, the 27 patients in our series underwent 35 NCT/CTA/PCT, TCD, and DSA examinations within 12 hours of each other (the imaging examinations separated by >12 hours from each other, especially the daily TCD examinations, were not considered). Eight patients underwent 3 successive imaging surveys (NCT/CTA/PCT, TCD, and DSA).
The NCT/CTA/PCT examinations were performed after a median delay of 7 days after the aneurysmal rupture (interquartile range, 6–9 days; range, 5–14 days). The TCD examinations were performed after a median delay of 7 days after the aneurysmal rupture (interquartile range, 6–8.5 days; range, 5–14 days). The DSA examinations were performed after a median delay of 7 days after the aneurysmal rupture (interquartile range, 6.5–9 days; range, 5–14.5 days).
With regard to the anatomic chart represented in Table 1, the results from the different imaging techniques were compared in each of the 630 (27 × 18) arterial territories in 27 patients. In this study, 402 arterial territories were DSA-negative and TCD-negative for vasospasm; 105 arterial territories were TCD-positive but DSA-negative (TCD false-positives); and 123 arterial territories were DSA-positive for vasospasm, 60 of which were endovascularly treated (Fig 1).
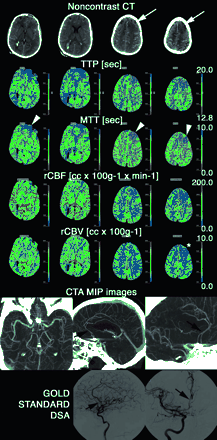
Patient transferred at day 8 to our neurovascular ICU from an outside institution after coiling of a ruptured ACom aneurysm. NCT obtained at the admission of the patient in our neurovascular ICU demonstrated extensive residual SAH and suspicious loss of gray-white matter contrast in the left superior frontal gyrus (white arrows). The tip of a right ventricular drain catheter is also visible. On PCT, significantly abnormal brain perfusion in the distribution of the anterior and inferior branches of the left (and also, to a lesser extent, right) ACA (arrowheads) and of the right posterior MCA branches is seen primarily on MTT and TTP maps. The rCBF was also slightly decreased in the same territories, whereas rCBV was mainly preserved (it is lowered only in the left superior frontal gyrus [star]). CTA confirmed the suspicion of moderate vasospasm of both A2 and A3 segments of the ACA (arrows), ultimately verified by gold-standard DSA. No abnormality of the right posterior MCA branches was identified. The artifacts created by the coils on the CTA images, obscuring the A1 segments bilaterally and interfering with their evaluation, are noteworthy. Endovascular therapy (intra-arterial verapamil) was performed in the ACA territories during the DSA.
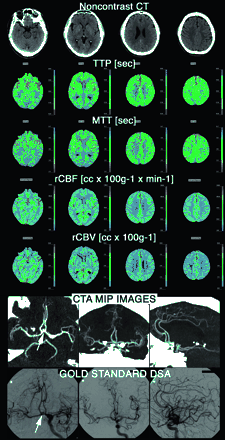
Patient transferred to our neurovascular ICU from an outside institution at day 4 after rupture of an ACom aneurysm (arrow). Admission TCD ultrasonography demonstrated increased absolute FV of 135 cm/s in ACAs. A CTA and PCT survey were obtained to rule out vasospasm; the PCT was completely normal and the CTA demonstrated the ACom aneurysm (arrow), but no vasospasm. DSA was performed determine the configuration of the aneurysm and to determine the best therapeutic strategy (endovascular coiling vs neurosurgical clipping). It confirmed the absence of vasospasm. TCD false-positive results were most likely related to the triple H (hypertensive, hypervolemic, hemodilutional) therapy undergone by the patient at the time of the TCD examination.
Twenty-five CTA examinations were considered of good quality. Two showed poor contrast bolus but were still interpretable. Eight showed significant metallic artifacts interfering with the interpretation of some segments of the circle of Willis, especially A1 segments, M1 segments, and PComs. These segments were considered negative, depending on the final DSA interpretation either CTA true-negative or false-negative.
Imaging Examinations Results
The measured values of the parameters in the 4 categories of arterial territories are reported in Table 2. Statistically significant differences could be identified between the 4 categories of arterial territories for all the PCT parameters (Kruskal-Wallis and Wilcoxon, P < .001) except rCBV.
Measured values of TCD and PCT parameters in the four categories of arterial territories defined according to DSA and TCD findings
With regard to the diagnosis of angiographic vasospasm, the PCT thresholds were set at the mid-value between the average PCT values in the group of DSA-negative arterial territories and in the group of DSA-positive arterial territories. The accuracy, sensitivity, specificity, NPV, and PPV of the different imaging techniques for the diagnosis of angiographic vasospasm were calculated. The thresholds and results are reported in Table 3.
Accuracy, sensitivity, specificity, NPV, and PPV of the different imaging techniques for the diagnosis of angiographic vasospasm
MTT with a threshold of 6.4 seconds was the most accurate parameter for the diagnosis of angiographic vasospasm, with a very high NPV of 98.7%.
In light of the high sensitivity of MTT and the high specificity of CTA, we evaluated the accuracy, sensitivity, specificity, NPV, and PPV of a combined interpretation involving first the assessment of MTT as a screening test and then the evaluation of CTA to confirm the diagnosis of vasospasm. The accuracy, sensitivity, specificity, NPV, and PPV of the MTT/CTA combination were 93.0%, 72.4%, 98.0%, 93.6, and 89.9%, respectively.
Regarding the endovascular treatment of vasospasm, the PCT thresholds were set at the mid-value between the average PCT values in the group of DSA-positive arterial territories without endovascular treatment and in the group of DSA-positive arterial territories with endovascular treatment. The accuracy, sensitivity, specificity, NPV, and PPV of the different imaging techniques for the diagnosis of vasospasm requiring endovascular treatment were calculated, with regard to DSA endovascular treatment as the gold standard. The thresholds and results are reported in Table 4.
Accuracy, sensitivity, specificity, NPV, and PPV of the different imaging techniques for the diagnosis of vasospasm requiring endovascular treatment
| Absolute FV (cm/s) | EICA ratios | |
|---|---|---|
| No vasospasm | <120 cm/s | <3.00 |
| Mild vasospasm | 120–139 cm/s | 3.00–3.99 |
| Moderate vasospasm | 140–179 cm/s | 4.00–5.99 |
| Severe vasospasm | >180 cm/s | >6.00 |
| BA/VA ratio | |
|---|---|
| No vasospasm | <2.70 |
| Mild vasospasm | 2.70–3.29 |
| Moderate vasospasm | 3.30–4.30 |
| Severe vasospasm | >4.30 |
MTT was again the parameter with the highest NPV, but with this time a threshold at 7.6 seconds, whereas PCT cortical rCBF with a threshold at 39.3 mL × 100 g−1 × min−1 was the most accurate indicator for endovascular therapy.
Discussion
The goal of this study was to evaluate whether a CTA/PCT combination could represent a noninvasive alternative to DSA for the diagnosis of vasospasm. In addition to the diagnostic value of the CTA/PCT combination, we evaluated its capability to predict which patients will require endovascular treatment. The hypothesis to be tested in future studies would be to first perform PCT/CTA in patients with suspected vasospasm and then perform angiography only in those patients who will undergo endovascular therapy. The combination of CTA and PCT seemed to be an ideal candidate, because CT is the imaging technique best suited for critically ill patients in the intensive care unit (ICU). Even if they cannot be performed at beside like TCD, CT examinations are frequently obtained as part of the standard of care in the follow-up of patients with ruptured intracranial aneurysms. They can be performed even in hemodynamically unstable patients. In these settings, obtaining PCT and CTA in addition to the NCT involves only the additional injection of a safe dose of iodinated contrast material.34
The present study demonstrates an excellent correlation of CTA and PCT results with those of DSA. The PCT values found in the different groups of patients, with and without angiographic vasospasm, are in agreement with previous reports in the literature.25
The most accurate noninvasive predictor of angiographic vasospasm was a combination of a sensitive MTT threshold of 6.4 seconds and CTA used as a specific confirmatory test. This interpretation strategy is characterized by a NPV of 93.6% and a PPV of 89.9%. Used alone, MTT with the same 6.4-second threshold achieved a very high NPV of 98.7%. Considering 100 patients—50 with vasospasm and 50 without vasospasm—if one applies the MTT criterion alone and performs DSA only in patients in whom a territory shows MTT values superior to 6.4 seconds on PCT, no patient would be inappropriately precluded from having an appropriate DSA. Fourteen patients without vasospasm would undergo invasive DSA, however, because of the relatively low specificity of MTT. By combining the MTT interpretation with CTA results, it would be possible to lower this number of unnecessary DSAs to 5.
Considered alone, CTA results lack sensitivity, especially for mild and moderate vasospasm, but are very specific. False-negative results and low sensitivity were contributed to by poor technique (2 cases of poor bolus in our series and 8 cases of metallic artifacts created by coils or clips in our series). Accuracy of CTA interpretation requires experience by the reviewer, specifically to distinguish between hypoplastic and vasospastic segments of the circle of Willis. Such distinction can be achieved by considering the segments adjacent to the questioned one. Typically, hypoplasia is suggested when adjacent segments appear normal, whereas vasospasm is suggested when adjacent segments are rather small in caliber. These criteria are not 100% accurate, however, which contributes to the false-positive CTA results. In this respect, evaluating the intracranial arterial segments on CTA in the light of the MTT information is very useful, because brain hemodynamics as reflected by MTT are altered in territories supplied by vasospastic arteries. The combined interpretation by using MTT and CTA reduces false-positive results for both techniques.
Using PCT cortical rCBF with a threshold at 39.3 mL × 100 g−1 × min−1 was the most accurate indicator for endovascular therapy (accuracy of 94.8%). If, however, the goal is to achieve the highest NPV (96.5%), in order not to miss any patient who could require endovascular treatment, MTT with a threshold at 7.6 seconds is optimal. This approach, however, would result in an unacceptable 62.8% false-positive rate. Therefore, the first strategy involving a rCBF threshold to select the patients who are more likely to undergo endovascular treatment is preferable, because it is associated with a reasonably low 3.9% false-negative rate and a lower 21.3% false-positive rate, respectively.
No statistically significant difference could be identified between the groups of patients with and without angiographic vasospasm as far as PCT cortical rCBV was concerned. Only the SD of the rCBV values was significantly higher in the group of patients with angiographic vasospasm. One possible explanation is that, in the group of patients with vasospasm, cerebral vascular autoregulation is often preserved and is thus associated with normal or increased rCBV values.35 The absence of significant difference in the rCBV values between the different groups of patients may explain why the thresholds for vasospasm diagnosis and endovascular treatment as previously defined were not discriminative and why the specificity of rCBV was poor.
PCT TTP was also not a discriminative parameter. This results from the fact that TTP values are calculated without any arterial input reference, as opposed to MTT. As a consequence, TTP is very sensitive to extracerebral or precerebral variables, such as cardiac function, aortic and aortic arch branch vessel stenosis and occlusion, or even variable position of the arm with variable impingement effect on the intravenous line where the iodinated contrast material bolus is injected for the PCT examination.
NCT hypodensities constitute a late finding after onset of vasospasm. They are thus characterized by high specificity—though false-positives can result from old lesions—but very low sensitivity, in agreement with previous reports.36
No correlation could be found between PCT and TCD results. Notably, no statistically significant difference in perfusion parameters was identified between patients with no vasospasm diagnosed on either DSA or TCD and patients with vasospasm diagnosed on TCD, but not on DSA (TCD false-positives). When compared with DSA, PCT was more accurate than TCD, the latter showing a significant number of false-positive and false-negative results. The sensitivity and specifity we report for TCD match the ones reported in the literature.11, 12
We acknowledge several limitations to our study. We selected conventional angiography as our gold standard because it is the standard test used in the routine medical practice. The distinction between the patients who underwent endovascular treatment and those who did not basically relied on the decision of performing an endovascular treatment, or not, made by the operator. This decision was made independently of the present study, in the best interests of the patient and according to the standard-of-care guidelines, by our team of interventional neuroradiologists, who have >20 years of experience in this field.
Our results remain to be verified in future, prospective longitudinal studies, where it will be possible to compare the CTA and PCT results measured when vasospasm is suspected to the results measured on admission. This will allow one to distinguish between vasospastic and hypoplastic arteries of the circle of Willis and also to assess the correlation between the evolution of PCT and DSA results with more significant statistical power.
Finally, we did not evaluate the vertebrobasilar territory in our study, because its perfusion territory was not assessed by PCT technique. Indeed, PCT is usually not used to assess the posterior fossa circulation, because of the streak artifacts related to the temporal bones and because of PCT limited spatial resolution. This constitutes a limitation of PCT technique. On the other hand, the CTA component of the vasospasm CT survey can assess the vertebrobasilar arteries. The value of CTA in the evaluation of vetebrobasilar vasospasm was recently reported in the literature.37
Conclusion
A CT survey with CTA and PCT represents an accurate screening evaluation of patients with suspected vasospasm and achieves better results than TCD. It can be used as a tool in selecting the best management strategy in these patients. On the basis of our results and by using the software described, we suggest the following interpretation strategy to more adequately select the patients for DSA. First, the MTT maps should be reviewed for arterial territories with MTT values superior to 6.4 seconds. These territories should be considered at risk for vasospasm. The corresponding artery supplying this territory should then be evaluated by CTA for vasospasm. If CTA of the corresponding artery is abnormal, the diagnosis of vasospasm is highly suggestive. The arterial territories at risk of or with positive vasospasm should be followed carefully and a decrease in cortical rCBF values should prompt DSA for possible endovascular treatment. This approach may help eliminate unnecessary invasive DSA in selected lower-risk patients and should be validated in future prospective studies.
References
- Received March 20, 2005.
- Accepted after revision June 1, 2005.
- Copyright © American Society of Neuroradiology