Abstract
BACKGROUND AND PURPOSE: Preoperative embolization of primary and metastatic spinal tumors is often performed to decrease intraoperative blood loss and facilitate surgical resection. The purpose of this study was to evaluate the safety of spinal tumor embolization and the variables that may influence intraoperative blood loss.
MATERIALS AND METHODS: A retrospective analysis of 100 spinal tumor embolization procedures was performed. Multiple variables were evaluated with respect to intraoperative blood loss, including tumor pathology, degree of tumor embolization, embolization above/below the levels involved, PVA particle size, surgical approach, and invasiveness.
RESULTS: There was 1 significant complication of the 100 embolization procedures performed. Evaluation of the entire set of embolization procedures demonstrated that RCC was associated with increased intraoperative blood loss (P = .009) relative to other tumor types, as were the surgical approach and invasiveness of the surgery performed. No other variables were found to be statistically significant predictors of intraoperative blood loss. Subset analysis of all RCCs demonstrated that complete embolization resulted in decreased blood loss compared with partial embolization (P = .03) and that male sex was associated with increased blood loss (P = .029).
CONCLUSIONS: Preoperative embolization of spinal tumors is a safe procedure. Complete embolization of RCCs results in lower intraoperative blood loss compared with partial embolization. The effectiveness of preoperative embolization of non-RCCs is unclear. Using smaller embolic particles and embolizing beyond the levels affected by tumor may not provide added benefit.
Abbreviations
- EBL
- estimated blood loss
- GCT
- giant cell tumor
- LR
- linear regression
- n/a
- not applicable
- PVA
- polyvinyl alcohol
- RCC
- renal cell carcinoma
- VEGF
- vascular endothelial growth factor
While primary tumors of the spine are relatively rare, metastatic disease involving the spine is common. It is estimated that 5%–10% of treatments of patients with cancer are complicated by spinal metastasis.1 Generally, treatment of spinal metastases may include radiation therapy, chemotherapy, and surgery. While surgery is often used for preserving neurologic function, stabilization of the spine, and local tumor control, it is often complicated by significant intraoperative blood loss.
Since the first description of selective transarterial embolization of spinal tumors by Benati et al in 1974,2 this procedure has been used preoperatively to decrease intraoperative blood loss and improve resectability, particularly for hypervascular tumors, such as RCCs. Multiple case series have demonstrated the effectiveness of preoperative embolization in reducing intraoperative blood loss.3–6
While preoperative embolization of RCC has become a standard of care, evidence for embolizing other types of tumors is scarce. Also, technical differences in the embolization procedure, such as the type of embolic agent used, embolizing above and below the tumor, partial-versus-complete embolization, and the time from embolization to surgery, have not been well evaluated. The purpose of this retrospective study was to evaluate the safety of spinal tumor embolization as well as the effects of tumor histology and the technical aspects of preoperative embolization on intraoperative blood loss in a series of 100 patients.
Materials and Methods
A retrospective review of medical records from the University of Washington and affiliated hospitals between June 1999 and December 2006 was performed on patients admitted for surgical management of spinal tumors undergoing preoperative embolization. Patients were identified from a data base of all patients undergoing spinal surgery. Permission was obtained from the institutional review board. Inclusion criteria were all patients who underwent spinal tumor embolization and had subsequent spinal surgery for treatment of the same tumor.
All patients undergoing spinal tumor embolization were placed under general anesthesia and monitored throughout the entire the procedure by the anesthesia department. Three neurointerventional surgeons performed all endovascular procedures by using a transfemoral approach. Diagnostic angiography of the pertinent spinal arteries by using a 5F Chuang II catheter (Cook, Bloomington, Indiana) was performed in sequential fashion. Following the diagnostic examination, selective catheterization of the pathologic arteries was performed by using a microcatheter. Involved levels without anterior or posterior spinal artery involvement underwent PVA particle embolization under continuous fluoroscopic examination. Gelatin sponge (Gelfoam; Phadia, Uppsala, Sweden), coils, or a combination was also used in the setting of potential collaterals necessitating flow diversion. Postembolization angiograms were obtained for all intervened vessels. The interventional records were reviewed for descriptions of the degree of tumor embolization: whether the embolization was complete or partial and whether levels above and below the tumor were embolized in addition to the levels involved with the tumor. The size of PVA particles infused was also recorded, with embolizations dichotomized into those using only <250 μm compared with those using all other sizes.
All surgeries were performed by the neurologic and orthopedic surgical faculty of the University of Washington with anterior, posterior, or combined approaches based on surgical preference. Intraoperative EBL and operative time were reported from the anesthesiology records. Operative notes were reviewed to determine the surgical approach used and to calculate the invasiveness index for each surgery. Briefly, the invasiveness index is a recently published method of comparing the extent of spinal surgical interventions by tabulating the interventions at each vertebral level.7 The 6 possible interventions include anterior decompression, anterior fusion, anterior instrumentation, posterior decompression, posterior fusion, and posterior instrumentation. Both the surgical approach and invasiveness index were used in the multivariate analysis of the data to correct the intraoperative blood loss for the degree of surgical intervention.
Medical records were also reviewed for patient age, sex, and final tumor pathology. Tumor extent was determined by using contrast-enhanced cross-sectional imaging (CT or MR imaging). Tumors were described according to location (cervical, thoracic, lumbar, or sacral) and the degree of bony involvement determined by the number of columns8 and levels.
Statistical analysis was performed on the entire dataset and the subsets of RCC and non-RCC tumors by using the SPSS software package (SPSS, Chicago, Illinois). Two-tailed Student t tests and Pearson correlations were calculated for individual variables relative to the EBL. In addition, LR analysis was performed, with inclusion of those variables approaching statistical significance (P = .05).
Results
A total of 100 spinal tumor embolization procedures were performed between June 1999 and 2006. Of these, 29 were primary tumors and 71 were metastatic tumors (Table 1). Twenty of these cases involved the cervical spine, whereas there were 63 cases involving the thoracic spine, 35 cases involving the lumbar spine, and 3 cases involving the sacrum. RCCs made up most (n = 38) of the metastatic tumors (Fig 1). Patients ranged from 16 to 88 years of age (mean, 54 years of age) and included 66 males and 34 females.
Tumor pathology
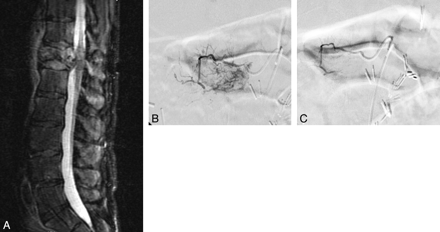
Embolization of a RCC metastasis. A, Sagittal short tau inversion recovery image demonstrates an expansile mass within the T12 vertebral body, which has invaded the spinal canal. B, Selective angiogram demonstrates hypervascular tumor blush at T12. C, Postembolization angiogram demonstrates no appreciable residual tumor blush.
One incident of an acute stroke was the only complication reported of the 100 cases of embolization. The patient experienced acute neurologic symptoms of dysmetria, gait imbalance, and ataxia after embolization of a RCC metastasis involving C6 to T1. MR imaging demonstrated a right cerebellar infarct. The patient's symptoms improved, and he was able to undergo spinal surgery 10 days following the embolization. Long-term morbidity is unknown because the patient was lost to follow-up. No instances of cord ischemia were reported.
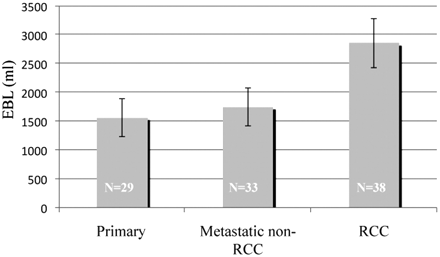
Analysis of the entire dataset showed mean EBLs for primary, non-RCC metastases, and RCC tumors of 1562, 1748, and 2856 mL, respectively (Fig 2). All non-RCC tumors combined had a mean EBL of 1664 mL, which was statistically significantly different from the EBL of RCC metastases (P = .014). No statistically significant difference between the mean EBL of tumors partially versus completely embolized was present (Table 2). Similarly, no significant differences were found in the EBL with respect to time from embolization to surgery, embolizing a level above/below the tumor, and PVA particle size. Tumors involving 3 columns or multiple levels had a mean EBL of 2384 mL compared with 1713 mL for less extensive tumors (P = .066). Evaluation of the surgical approach showed that a combined approach resulted in greater intraoperative blood loss compared with either a posterior or anterior approach (P = .017). Similarly, a greater invasiveness index was highly correlated with increased EBL (P = .004). Differences in EBL were originally statistically significantly greater (P = .037) for males compared with females; however, regression analysis showed that this did not persist.
Graph shows EBL versus tumor type.
EBL for all tumors
The subset of 38 RCCs contained 30 males and 8 females (Table 3). Differences in mean EBL between males (3310 mL) and females (1156 mL) were statistically significant (P = .001). Comparison of partially embolized (3460 mL) with completely embolized (1821 mL) tumors was also statistically significant (P = .028). Differences in EBL with respect to embolizing above/below the involved level showed a trend toward decreased EBL in those cases in which embolization was performed above/below (2068 mL) versus those cases in which it was not (3178 mL); however, this was not statistically significant (P = .182). Results of the evaluation of PVA particle size and the time interval to surgery with respect to differences in EBL were not statistically significant. Tumors involving 3 columns or multiple levels had a mean EBL of 3218 mL compared with 1845 mL for less extensive tumors (P = .064). Evaluation of the surgical approach yielded EBLs of 3720, 2489, and 1008 mL for combined, posterior, and anterior approaches, respectively. The difference in mean EBLs between the combined surgical and posterior and anterior approaches was not statistically significant (P = .131). Similarly, the Pearson correlation of the invasiveness index did not demonstrate a statistically significant relationship with EBL (P = .49). LR analysis showed that only sex and degree of embolization were statistically correlated with EBL.
EBL for a subset of RCC tumors
Subset analysis of non-RCC tumors demonstrated no statistically significant differences in EBL with respect to sex, partial-versus-complete embolization, embolizing a level above/below the involved level, PVA particle size, and time interval to surgery (Table 4). Evaluation of the surgical approach demonstrated greater EBL from a combined approach (2275 mL) compared with either a posterior (1487 mL) or anterior (1149 mL) approach (P = .001). Similarly, the invasiveness index was highly correlated with EBL (P = .001).
EBL for a subset of non-RCC tumors
Discussion
Preoperative spinal tumor embolization is often used to decrease intraoperative blood loss and improve resectability. The literature on preoperative spinal tumor embolization predominantly comes from case reports and retrospective series, most describing cases of RCC. To our knowledge, no randomized controlled studies have been performed to date. This study is the largest retrospective cohort representing a wide range of tumor pathologies, though metastatic tumors, primarily RCC, were the most common.
Spinal tumor embolization carries a certain amount of risk, including complications of vascular access (eg, hematoma or pseudoaneurysm), radiation exposure, iodinated contrast, catheter manipulation (eg, vessel dissection or rupture), or embolization (eg, spinal or cerebral infarction). As with previous studies, embolization proved safe.9,10 Only a single major complication, a cerebral infarction, occurred during the diagnostic portion of a cervical embolization. There were no cases of spinal infarction. While there was only 1 documented complication from embolization in our case series, this may not reflect the true complication rate. Our case series only included patients who underwent embolization and then subsequently underwent surgery. It is conceivable that a patient may have had a complication from embolization and then did not undergo surgery, thereby being excluded from our analysis. Also, in patients in whom surgery occurred soon after embolization, a potential complication may not have been recognized before surgery but then was thought to be a complication of surgery rather than embolization.
Our data indicate that primary tumors and non-RCC metastasis had mean blood losses similar to those of the literature benchmark of approximately 2 L.3–5,11–13 RCCs demonstrated a statistically significantly greater blood loss with EBLs approaching 3 L (P = .009). This value is higher than those reported by others; though after subdividing these cases into those undergoing complete or partial embolization, the mean EBL of completely embolized RCCs approximated a value just under 2 L, an amount comparable with that in these previous studies.
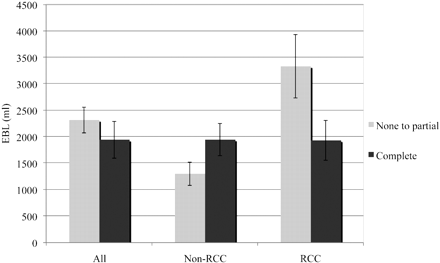
While no matched nonembolized controls were available, dividing the cases into partial versus complete embolization may provide a useful analysis of the importance of complete embolization. Reasons for incomplete embolization were most commonly the presence of nearby radicular arteries communicating with the anterior spinal artery. The subset of RCCs showed that complete embolization resulted in a statistically significant decrease in EBL; whereas in the non-RCC group, completely embolizing a tumor resulted in no statistically significant difference in EBL compared with partial embolization (Fig 3). This difference suggests that the utility of embolizing non-RCC spinal tumors is unclear. However, there are likely additional types of tumor pathologies that may also respond to embolization that could not be individually analyzed in this study due to their limited representation in this dataset.
Graph shows partial versus complete embolization.
To improve interventional practice, we wanted to examination how common technical variables of embolization practice affect blood loss. We looked at 2 concepts concerning embolization: 1) The more levels that are embolized, the greater is the decrease in blood loss; and 2) smaller particles (PVA < 250 μm) achieve more distal embolization within the tumor vascular bed and, in turn, have a greater effect on decreasing blood loss. For many patients, embolization was performed above and/or below the involved spinal level in anticipation of either complete or partial resection of those levels, depending on surgical necessity. The RCCs showed a strong trend toward decreased blood loss with embolization of additional levels, though the difference did not meet statistical significance; whereas no significant difference was noted for non-RCCs. Comparing PVA particle size, we noted no significant difference in EBL between sizes in either subgroup, similar to what has been shown previously.6 This is important because smaller particles, though able to migrate farther in a vascular bed, carry the added risk of nontarget embolization.
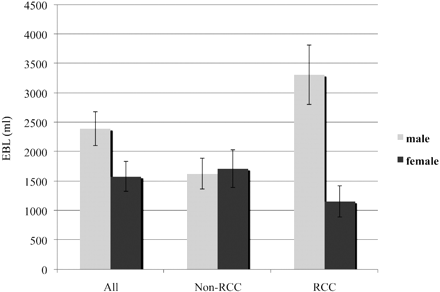
An unexpected, and to our knowledge, previously unpublished result is the apparent effect of sex on intraoperative blood loss in patients with RCC. In our subset of 38 patients with RCC, males had a statistically significant greater blood loss compared with females, which persisted when correcting for confounding variables, such as tumor extent, surgical approach, and invasiveness (Fig 4). It is also interesting that neither the surgical approach nor the invasiveness index seemed to correlate with EBL, whereas these variables were a significant predictor of blood loss in non-RCC surgeries. A recently published review of 35,336 RCC cases showed that males present with larger and higher grade tumors compared with females.14 While we attempted to correct our results for tumor size by using tumor extent, we did not correct our data for tumor grade. Other authors have shown that VEGF expression is correlated with RCC tumor grade, so that higher grade tumors express more VEGF compared with lower grade tumors.15 Since VEGF is known to play an important role in angiogenesis and males generally present with higher grade tumors, this correlation may be a possible explanation for the greater intraoperative blood loss in male patients, though more research would be required to elucidate such a relationship.
Graph shows EBL versus sex.
Limitations to our study are primarily a result of its retrospective design. While we attempted to control for multiple possible confounding variables, such as surgical approach and invasiveness, as well as multiple embolization procedural variables, there may be additional variables that were not accounted for. A randomized controlled trial would be useful, perhaps not to bolster the practice of embolizing RCCs but certainly for other pathologic types such as adenocarcinoma or squamous cell carcinoma metastasis, the incidence of which makes data collection and clinical application practical. Another limitation of our study was the large variety of tumor types that were embolized. While the utility of preoperative embolization of RCCs for reducing intraoperative blood loss has been demonstrated in multiple case series, limited data are available on embolization of non-RCC spinal tumors. Although we collected data on 63 non-RCC cases, they represented a wide range of tumor pathology, limiting our ability to elucidate potential differences in response to embolization of tumors in which only a few cases were represented. There have been other case series looking at the role of embolization for a single non-RCC pathologic type demonstrating the safety and beneficial effect on blood loss, but these are small in sample size and all retrospective in design.16–22
Conclusions
Spinal malignancy, primary or secondary in nature, can present a surgical challenge due to intraoperative blood loss. Preoperative embolization is a safe and effective means to decrease intraoperative blood loss for RCCs. Its effectiveness in decreasing intraoperative blood loss for non-RCC spinal tumors is unclear, and further research is needed to elucidate its potential role for specific tumor types. The practice of using smaller embolic particles (<250 μm) and embolizing beyond the effected levels may not provide added benefit in decreasing blood loss.
Acknowledgments
We thank Jeffery Jarvik, Mark Konodi, William Kreuter, and William Hollingworth for the assistance with statistical analysis and the members of the radiologic, orthopedic, and neurosurgical departments for their hard work.
Footnotes
-
Paper previously presented in part at: Annual Meeting of the Society of Neurointerventional Surgery, July 27–31, 2008; Lake Tahoe, California.
References
- Received April 23, 2009.
- Accepted after revision August 27, 2009.
- Copyright © American Society of Neuroradiology