Abstract
BACKGROUND AND PURPOSE: CAS carries an inherent risk of distal cerebral embolization, precipitating new brain ischemic lesions and neurologic symptoms. Our purpose was to evaluate the frequency of new ischemic lesions found on DWI after protected CAS placement and to determine its association with plaque morphology.
MATERIALS AND METHODS: Fifty patients (mean age 65.13 ± 7.08 years) with moderate and severe internal carotid artery stenosis underwent CAS with distal filter protection. Fibrolipid and fibrocalcified plaque morphology was determined by sonography according to the relative contribution of echogenic and echolucent material, and by multisection CT using plaque attenuation. There were 46.81% of patients with fibrolipid and 53.19% with fibrocalcified plaques. DWI was performed before and 24 hours after CAS.
RESULTS: Seven (14.89%) patients showed new lesions. Four (8.51%) had 6 new lesions inside the treated vascular territory. Three had a single lesion and 1 patient had 3 lesions (mean: 1.5 ± 1). Most lesions (66.66%) were subcortical, with a mean diameter of 9 mm (range 5–15 mm). All lesions occurred in the area supplied by the middle cerebral artery and were clinically silent. A significant relationship was found between plaque morphology and the appearance of new lesions. Patients with fibrolipid plaques had a significantly higher number of new lesions compared with patients with fibrocalcified plaques (P = .041). The absolute risk of new lesions in the fibrolipid group was 18.18%.
CONCLUSIONS: New ischemic lesions were observed in the treated vascular territory in 8.51% of patients. The appearance of new ischemic lesions was significantly related to the plaque morphology. Fibrolipid plaques were associated with higher numbers of new lesions.
ABBREVIATIONS:
- ACT
- activated coagulation time
- CAE
- carotid artery endareterectomy
- CAS
- carotid artery stenting
- DUS
- Doppler ultrasound
- HU
- Hounsfield unit
- PSV
- peak systolic velocity
CAS carries an inherent risk of distal cerebral embolization, precipitating new brain ischemic lesions and neurologic symptoms.1⇓⇓–4 This has led to the development and widespread application of cerebral protection devices.5,6 The most widely used devices are those based on distal filter placement that capture emboli dislodged from plaque; however, their application may result in additional complications.7⇓⇓⇓⇓–12
Several reviews have reported contradictory data concerning the rate of stroke and ischemia after protected versus unprotected stent placement.3,13⇓–15 The frequency of new ischemic lesions after CAS may be associated with numerous factors, such as clinical status, vascular anatomy, plaque morphology, and complexity. Therefore, the need to identify patients at risk for embolic events has become increasingly important. The morphologic characteristics of atherosclerotic carotid plaques may be useful in heralding embolic potential in the carotid arteries. Several authors have reported that plaques in the carotid arteries that are associated with large lipid pools or soft extracellular lipid are more prone to rupture and production of emboli.16 DWI is the most sensitive tool for the detection of neurologically silent or asymptomatic infarcts at a very early stage.17⇓–19
The aim of this study was to determine the frequency of new ischemic DWI lesions in patients with moderate and severe ICA stenosis after protected CAS using a filter device, and to determine its potential association with plaque morphology.
Materials and Methods
Study Population
From June 2009 to May 2010, 50 patients with moderate (50%–69%) and severe (70%–99%) ICA stenosis were treated with protected CAS following a prospective protocol. The severity of carotid stenosis was calculated according to NASCET criteria.20 The carotid stenosis was considered symptomatic if the patient had experienced an ipsilateral TIA or nondisabling stroke (modified Rankin Scale ≤2). Symptomatic patients usually underwent the procedure 2–3 weeks after the diagnosis of the ICA stenosis, and no later than 6 weeks after the initial observation. In 4 patients, the procedure was performed on an emergency basis.
Asymptomatic patients were included if stenosis was 80% by DUS or if silent lesions were documented on CT/MR. All patients were unsuitable for CAE because of a previous CAE, occlusion of the contralateral ICA, bilateral severe ICA stenosis, rigid or short neck, neck irradiation, or risk factors for surgical complications. Three patients were excluded from the study due to pacemaker implantation (1 patient) and technical reasons (2 patients). Age, sex, vascular risk factors, and neurologic history and status of each patient were recorded before the procedure. Vascular risk factors included hypertension, diabetes, hyperlipidemia, smoking (past or present), coronary heart disease, and peripheral artery disease.
The study was approved by our local ethics committee. All patients gave their written informed consent before participation in the study.
Patient Assessment
Neurologic examinations were performed by an independent team of 3 neurologists with special expertise in stroke. Before treatment, all patients underwent careful neurologic examination, B-mode sonography, DUS, and CTA. The neurologic examination and complete sonography/DUS evaluation were performed within 24 hours of the procedure and 30 days after the procedure.
Carotid Plaque Characterization
Plaque morphology, defined as predominantly fibrolipid or fibrocalcified, and degree of stenosis were initially determined by sonography/DUS evaluation and CTA of supra-aortic vessels during the inclusion period. All sonography/DUS examinations were performed by a single experienced neurosonographer using high-resolution MyLab70 (Esaote, Genova, Italy) with a 7.5–10.00 MHz linear probe. B-mode sonography images of the ICA were obtained in transverse and longitudinal directions to determine the maximum extent of the atherosclerotic plaque.
The classification of Gray-Weale21 was used for sonography plaque characterization as follows: Type I, predominantly echolucent plaque with a thin echogenic cap; Type II, substantially echolucent lesions with small areas of echogenity; Type III, predominantly echogenic lesions with small areas of echolucency; and Type IV, uniform echogenic lesions (equivalent to homogeneous). Type I and Type II were predominantly soft plaques with fibrolipid structure; Type III and Type IV were predominantly fibrotic and partly calcified plaques (hard plaques). In our study, Types I and II were classified as fibrolipid plaque (Fig 1), and Types III and IV as fibrocalcified plaque (Fig 2). The severity of carotid stenosis was evaluated by measuring the PSV, with angle correction, at the narrowest point of stenosis. A stenosis was classified at more than 70% if PSV was more than 200 cm/s. The neurosonographer was not aware of the DWI findings.
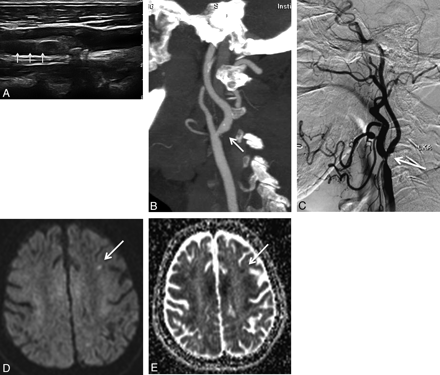
A 69-year-old man with severe ICA stenosis. B-mode sonography (A), CTA (B), and DSA (C) show severe stenosis of the left ICA caused by fibrolipid plaque (arrow). DWI (D) shows a frontal subcortical high signal intensity lesion (arrow). On ADC map (E), the lesion shows low signal intensity (arrow) indicating its acute nature.
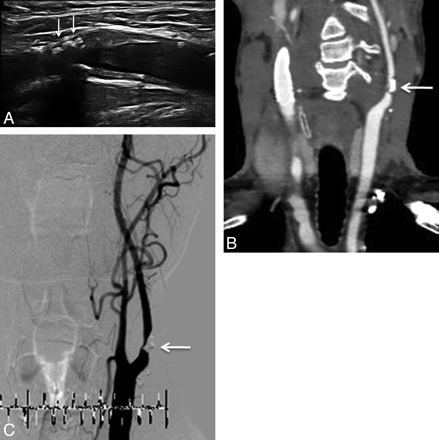
A 64-year-old man with severe ICA stenosis without new ischemic lesions after CAS. B-mode sonography (A), CTA (B), and DSA (C) show severe stenosis of the left ICA caused by fibrocalcified plaque (arrow).
CTA was performed on all patients using an Aquillion 64 MDCT scanner (Toshiba Medical Systems, Tokyo, Japan). After a delay determined by an automated bolus-timing program, an injection of 80 mL of nonionic contrast medium was performed at a rate of 4 mL/s. The CT scan was then performed from the aortic arch to the supraventricular white matter. The image data were transferred to a Vitrea 2 workstation (Toshiba Medical Systems) for postprocessing. Plaque type was classified according to attenuation measurements from the previously reported criteria.22 Predominantly lipid or fibroid plaques were defined as soft, intermediate plaques with a median attenuation of ≤130 HU (Fig 1). Calcified plaques consisted of lesions having a median attenuation >130 HU (Fig 2). When there was a disagreement in plaque characterization between CTA and DUS, we used data obtained by CTA.
Medication
A combination of aspirin (100 mg/day) and clopidogrel (75 mg/day) was administered 7 days before the procedure. Patients were loaded with 300 mg of clopidogrel if they had not received it before the procedure.
During CAS, heparin was administered before intra-arterial catheterization to maintain an ACT between 250 and 300 seconds. Just before the poststenting dilation phase, atropine (0.5–1 mg IV) was given to most patients to reduce the bradycardia and hypotension potentially associated with carotid dilation. Patients were discharged on a regimen of clopidogrel (75 mg/day) for 6 weeks and aspirin (100 mg/day) for the remainder of their lives.
Interventional Protocol
All procedures were performed by the same interventional radiologist. Angiography of the treated carotid artery, or both intra- and extracranial carotid arteries, was performed in case of disagreement between DUS and CTA. The procedures were performed using a right femoral approach in 44 patients and a right brachial approach in 3 patients. We treated stenosis of the right ICA in 18 patients (38.3%) and left ICA in 29 patients (61.7%). Under digital road-mapping, a 0.035″ stiff guidewire (Extrastiff Glide; Terumo, Tokyo, Japan) gently placed into the ipsilateral external carotid artery was then used to advance a 90-cm 6F sheath catheter (Destination, Terumo) into the common carotid artery. The atherosclerotic lesion was then gently bypassed by the protection device, which was deployed distal to the lesion into a strait segment of the ICA, extracranially, to maintain cerebral perfusion and capture any debris dislodged during the intervention. The types of filters used were as follows: AngioGuard (Cordis, Miami Lakes, Florida) in 24 patients (51.06%), Spider RX (ev3, Plymouth, Minnesota) in 20 patients (42.56%), and TwinOne (Minvasis, Gennevilliers, France) in 3 patients (6.38%).
Predilation of the ICA lesion was performed in 16 patients (34%) using either a 3 × 20 mm, 3.5 × 20 mm, or 4 × 20 mm balloon catheter with single inflation. A self-expandable stent was then navigated over the wire of the filter and placed across the stenotic segment. The type of stent placed was as follows: Wallstent (Boston Scientific, Natick, Massachusetts) in 21 patients (44.68%), Protégé (ev3) in 22 patients (46.81%), and Precise (Cordis) in 4 patients (8.51%). In 1 patient (2.13%), 2 stents were placed. The size of the stent was determined during the procedure. Dilation after stent placement was performed using a 6 × 20 mm balloon catheter in 44 patients (93.62%).
Distal protection devices and stents were selected according to the preference of the interventional radiologist performing the procedure and commercial availability.
MR Imaging Technique
MR imaging scans were obtained immediately before and 1 day after CAS using a 1.5T Avanto scanner (Siemens, Erlangen, Germany). Our imaging protocol included transverse T2-weighted spin-echo (TE: 93; TR: 4700; section thickness: 5.0 mm; matrix size: 256 × 320; gap: 0; FOV: 230; number of excitations: 2), FLAIR (TE: 111; TR: 8840; TI: 2500; section thickness: 5.0 mm; matrix size: 256 × 256; gap: 0; FOV: 210; number of excitations: 2), and DWI (TE: 99; TR: 3600; b = 1000 seconds/mm2; section thickness: 5.0 mm; gap: 0; matrix size: 128 × 128; FOV: 230; number of excitations: 3). ADC maps were obtained in all cases. The same scanner and the same imaging parameters were used throughout the study.
Two neuroradiologists analyzed all scans. Disagreement was resolved by consensus. The pretreatment and posttreatment scans were compared. DWI scans and ADC maps were used to detect new acute ischemic brain lesions. On each scan, the number, distribution (cortical, subcortical, or deep white matter), and vascular and anatomic distribution were recorded. Lesions were classified into 3 groups according to size (<5 mm, 5–10 mm, >10 mm). Lesions were considered separate if there was no continuity between them on the same section as well as on adjacent sections.
Statistical Analysis
Data were presented as absolute numbers, percentages, and mean ± SD. Categoric variables were compared by χ2 test or Fisher exact test, where appropriate. Logistic regression analysis was performed to assess the independent association between the occurrence of new DWI lesions with baseline clinical and angiographic characteristics, and the types of stents and protection devices used. Interrater agreement regarding plaque characterization and percent stenosis was determined using the Cohen κ statistic. A P value <.05 was considered significant. Data were analyzed using the SPSS software package (SPSS, Chicago, Illinois).
Results
Demographic and clinical characteristics of the study population are summarized in Table 1. Forty-seven patients (27 men and 20 women) participated in the study. The mean age was 65.13 ± 7.08 years (age range 50–79 years). Regarding baseline characteristics, vascular risk factors, and degree of stenosis, there were no significant differences between the subgroups of patients with and without new lesions in the treated vascular territory.
Baseline patient demographics
All procedures were successfully completed with no postprocedural neurologic complications. DWI showed a total of 15 new lesions in 7 patients (14.89%). Six new DWI lesions (mean 1.5 ± 1 per patient) were located within the vascular territory of the treated artery in 4 patients (8.51%; Table 2). Three patients (6.38%) had 9 new lesions (mean 3 ± 1.26 per patient) outside of the treated vascular territory. Two patients developed only contralateral lesions. One patient had bilateral lesions.
Patients with new DWI lesions inside the treated vascular territory
Three patients had a single lesion and 1 patient had 3 lesions inside the treated vascular territory. Most were subcortical (66.66%). There was a single cortical/subcortical lesion (16.66%) and a single deep lesion (16.66%). According to size, most lesions were in the range of 5–10 mm (4; 66.66%). Two lesions (33.34%) were larger than 10 mm. The mean diameter of the ipsilateral lesions was 9 mm (range: 5–15 mm). All lesions occurred in the area supplied by the middle cerebral artery.
We found a significant relationship between plaque characteristics and the appearance of new lesions. Patients with fibrolipid plaques had a significantly higher number of new lesions compared with patients with fibrocalcified plaques (P = .041). The absolute risk of new lesions in patients with fibrolipid plaques was 18.18%.
No significant relationship was found between the rate of new ischemic lesions in the treated vascular territory and CAS-related factors. There was no association between the incidence of new lesions in the treated vascular territory and the type of filter used (Spider RX in 3 patients and TwinOne in 1 patient; P = .735) as well as the type of the stent used (Protégé in 3 patients and Wallstent in 1 patient; P = .474). The predilation (P = .580) and postdilation (P = .761) procedures were not associated with the appearance of new lesions in the treated territory. No significant relationship was found between the rate of new lesions in the treated vascular territory and variables such as age (P = .324), clinical presentation (P = .246), or vascular risk factors (P = .658). The severity of stenosis was not related to the incidence of new lesions in the treated vascular territory (P = .659). The number of patients, however, was too small to allow investigation using a full regression model, though association between occurrence of new DWI lesions and baseline clinical and angiographic characteristics, or between different types of stents and filter devices, was not significant.
Interrater agreement between the 2 methods (DUS and CTA) was 95.7% (Cohen κ = 0.957) for plaque characterization. Interrater reliability of percent stenosis characterization was only 14.2% (Cohen κ = 0.142).
One-Month Follow-Up
In 1 patient, neurologic examination showed a TIA on the side of the stent with no new DWI lesions. One patient died of myocardial infarction 3 days after stent placement; however, DUS showed no recurrent stenosis or occlusion of the stent.
Discussion
Several investigators, using DWI to detect clinically silent embolic lesions after protected CAS, have reported new DWI lesions over a wide range, from 17.3% to 73%.1,3,4,6,10,13⇓–15,23⇓⇓⇓⇓–28 The rate of new DWI lesions in our study was lower compared with these other studies. Of 47 patients, 14.89% had new lesions after CAS with distal filter protection, and new ischemic lesions were detected in the treated vascular territory in 8.51% of patients.
A lower incidence of new DWI lesions after protected CAS (8.33%) was published in only 1 other study with fewer patients.29 Several systemic reviews have reported contradictory data concerning the rate of stroke and ischemia using protected versus unprotected CAS. The rate of new DWI lesions in our study was lower compared with unprotected CAS. Previous reports revealed rates of new lesions after unprotected CAS ranging from 18% to 68%.3,5,13,14,30⇓⇓⇓–34 Our findings are in agreement with other observational studies reporting lower rates of stroke and ischemia on DWI with protected stent placement compared with unprotected stent placement.2,3,13,15 These findings cast doubt on the efficacy of routine use of filter-type cerebral protection devices. A randomized trial comparing protected versus unprotected stent placement is needed to investigate the safety and efficacy of cerebral protection devices.
The mean number of new lesions inside the treated vascular territory in our study (1.5 ± 1 per patient) was in agreement with the reported incidence of between 1.42 and 2.8.1,10,28,35⇓⇓–38 Most were at the cortico-subcortical junction. The size, subcortical location, and distribution in the vascular territory supplied by the treated artery were indicative of their embolic origin. The rate of detected lesions may have been affected by the size of ischemic lesions and type of DWI technique used. Most reported studies were performed using standard DWI with a section thickness of 5–6 mm. A partial volume effect due to the presence of CSF signal intensity, and lack of contrast between the lesion and the surrounding normal brain parenchyma, may have reduced detectability on standard DWI, as most new lesions occurred in the cortical and subcortical areas.31
Thin-section DWI (section thickness 2 mm) is able to detect small cortical lesions better than standard DWI. Yamatogi et al39 reported a higher rate of lesions (80%) located on the ipsilateral side of the treated ICA detected on thin-section DWI. The mean diameter of these lesions (5.16 mm) was lower compared with our study (9 mm). New asymptomatic ischemic lesions might lead to clinical consequences over the long term, including cognitive decline and dementia.26 Detection of small ischemic lesions after CAS may be important for patient prognosis.
We found a significant relationship between plaque morphology and the appearance of new lesions. Patients with fibrolipid plaques had a significantly higher number of new lesions compared with patients with fibrocalcified plaques. The absolute risk of new lesions in patients with fibrolipid plaques was 18.18%. Our results are in agreement with the study of Cremonesi et al.40 In this study, all patients with embolic complications after protected CAS had soft, uniformly echolucent, or predominantly echolucent plaques at DUS evaluation. This pattern may have developed because fibrolipid plaques are less stable and more vulnerable compared with fibrocalcified plaques. The vulnerable atherosclerotic plaque is characterized by an increased tendency to rupture, resulting in embolization or thrombosis.41 Several authors have reported that plaques in the carotid arteries associated with large lipid pools or soft extracellular lipid are more prone to rupture and, consequently, produce emboli.42⇓⇓⇓–46
The role of calcification in atherosclerotic disease, with regard to clinical symptoms, has been investigated using both pathologic and sonographic studies. Calcium is postulated to confer stability by stiffening the plaque, resulting in protection against biomechanical stress and subsequent disruption.42 In a clinical pharmacologic study of endarterectomy specimens from asymptomatic and symptomatic patients, Hunt et al43 found that patients with calcified carotid plaques had fewer cerebrovascular events than those with noncalcified plaques. On B-mode sonography, echo-rich carotid plaques are typically associated with calcium and fibrous tissue and echolucent plaques are associated with high lipid content. The Tromso study,44 using this method, found that echo-rich plaques carry a lower risk of neurologic symptoms. Nandalur et al45 reported that extracranial carotid artery calcified plaques causing stenosis are less likely to be symptomatic and thus may be more stable than noncalcified plaques. The increased instability of fibrolipid plaques might pose a greater risk for cerebral embolism with protected CAS.
We assume that all intra-arterial manipulations carry a certain risk for embolism and subsequent ischemic events. Particulate plaque debris can be spread during the initial wire access, the insertion of the filter (especially in tortuous vessels and stenosis that are difficult to pass), predilation, stent deployment, and technical misadventures made in an attempt to retrieve the filter after stent deployment. Some atherosclerotic particles may pass through the filter pores or between the filter basket and the vessel wall, especially when an autoexpandable filter with a fixed size is used. The withdrawal of the filter protection system may squeeze captured atherosclerotic materials from the filter basket into the treated vessel.
We assumed that the total number of new lesions in the treated territory was not related exclusively to the CAS procedure. Emboli may source from guiding catheter placement, guidewire introduction, injection of contrast medium, or catheter flushing before the filter deployment.10,26
DWI lesions outside the treated vascular territory were not caused by the treatment for stenosis. The rate of new lesions outside the treated territory in our study (6.38%) was lower compared with previous studies using diagnostic angiography and CAS.10,15,47
We did not find any association between the incidence of new DWI lesions in the treated vascular territory and the different types of filter protection devices used in our study. Our results, however, cannot be generalized to all filters available, as filters can differ slightly in pore size, as well as nature (concentric versus eccentric), and deployment mechanism. In a recent large study involving more than 3000 patients, the type of embolic protection device did not influence the clinical outcome after CAS.48 Future studies should clarify the effects of different types of cerebral protection devices on the incidence of new DWI lesions after CAS.
At 1-month neurologic follow up, TIA was reported in 1 patient from the group, with no new DWI lesions on the stent placement side. One patient died of myocardial infarction 3 days after stent placement. Short-term results in our study were acceptable with respect to neurologic events. The rate of neurologic events in our patients was lower in comparison with other studies.15
Limitations
The primary limitation of this study was the small number of patients who underwent protected CAS.
Conclusions
New ischemic DWI lesions were observed in 14.89% of patients after filter-protected CAS. In 8.51% patients, lesions were located inside the treated vascular territory. We found a significant increase in new DWI lesions in the treated vascular territory in patients harboring fibrolipid versus fibrocalcified plaques. The absolute risk of new lesions in treated vascular territory for patients with fibrolipid plaques was 18.18%.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received March 15, 2011.
- Accepted after revision July 18, 2011.
- © 2012 by American Journal of Neuroradiology