Abstract
BACKGROUND AND PURPOSE: Current MRI with the CE T1-weighted sequence plays a limited role in the evaluation of facial neuritis due to prominent normal facial nerve enhancement. Our purpose was to retrospectively investigate the usefulness of the CE 3D-FLAIR sequence compared with the CE 3D-T1-FFE sequence in facial neuritis patients.
MATERIALS AND METHODS: We assessed 36 consecutive patients who underwent temporal bone MR imaging at 3T for idiopathic facial palsy. Two readers independently reviewed CE 3D-T1-FFE and CE 3D-FLAIR images to determine the degree of enhancement in each of 5 segments of the facial nerve. We compared AUCs using the Z-test, compared diagnostic performance of 2 MR techniques with the McNemar test, and evaluated interobserver agreement. The Pearson χ2 test was used for each segment of the facial nerve.
RESULTS: The AUC of CE 3D-FLAIR (reader 1, 0.754; reader 2, 0.746) was greater than that of CE 3D-T1-FFE (reader 1, 0.624; reader 2, 0.640; P < .001). The diagnostic sensitivities, specificities, and accuracies were 97.2%, 86.1%, and 91.7%, respectively, for CE 3D-FLAIR, and 100%, 56.9%, and 78.5%, respectively, for CE 3D-T1-FFE. The specificity and accuracy of CE 3D-FLAIR were greater than those of CE 3D-T1-FFE (specificity, P = .029; accuracy, P = .008). The interobserver agreements for CE 3D-FLAIR (κ-value, 0.831) and CE 3D-T1-FFE (κ-value, 0.694) were excellent. Enhancement of the canalicular and anterior genu segments on CE 3D-FLAIR were significantly correlated with the occurrence of facial neuritis (P < .001 for canalicular; P = .032 and 0.020 for anterior genu by reader 1 and reader 2, respectively).
CONCLUSIONS: CE 3D-FLAIR can improve the specificity and overall accuracy of MR imaging in patients with idiopathic facial palsy.
ABBREVIATIONS:
- AUC
- area under curve
- AVP
- arteriovenous plexus
- CE
- contrast- enhanced
- CNR
- contrast-to-noise ratio
- EPS
- electrophysiologic study
- FFE
- fast-field echo
- FSPGR
- fast-spoiled gradient recalled
- Gd-DTPA
- gadolinium-diethylene-triamine pentaacetic acid
- ROC
- receiver operating characteristic
- SENSE
- sensitivity encoding
- VISTA
- volumetric isotropic T2-weighted acquisition
MR imaging of patients with facial neuritis is usually not indicated except for patients with facial palsy who have atypical manifestations and those with intractable palsy despite therapy. In most patients, the diagnosis of facial neuritis is clinically evident and EPS confirms this. EPS can also provide prognostic information on outcomes in patients with acute facial palsy. However, the major disadvantage of EPS is its inability to detect diagnostic abnormalies of the nerve distal to the stylomastoid foramen within 1 week of symptom onset.1
Although the potential of MR imaging has been studied as part of the effort to find other helpful diagnostic techniques over the past 20 years,2⇓⇓⇓⇓⇓⇓⇓–10 its results have been largely disappointing. Gd-DTPA-enhanced T1-weighted spin-echo and 3D spoiled gradient-echo sequences have shown significant limitations in diagnosing and predicting outcomes in patients with facial neuritis; the geniculate ganglion, tympanic, or mastoid segment of the normal facial nerve can be significantly enhanced in up to 76% of patients due to the distribution of arteriovenous plexus along the facial nerve, which, in turn, may inhibit evaluation of the pathologic enhancement of the nerves resulting from breakdown of the blood nerve barrier.10⇓–12 Thus, MR imaging plays a limited role in the diagnosis and provision of prognostic information of facial neuritis.3,7
Recently, a new isotropic 3D-T2-weighted imaging technique, the 3D turbo spin-echo sequence with variable flip angles (VISTA, sampling perfection with application optimized contrast using different flip angle evolution [SPACE], or Cube), was introduced on the 3T MR system.13 This sequence uses variable refocusing flip angles to constrain T2 decay over a long echo train, with minimal blurring, and can acquire isotropic resolutions in a clinically acceptable scan time. When applied to the temporal bone pathologies, this 3D-FLAIR sequence had higher SNR and CNR than previous 2D sequences.14,15 Moreover, the 3D-FLAIR sequence has been shown useful in the diagnosis of mumps-related deafness and sudden sensorineural hearing loss, and for predicting the prognosis of patients with sudden sensorineural hearing loss, which is not usually demonstrated by the conventional MR imaging sequences.16⇓–18 This ability of the 3D-FLAIR sequence to find minute abnormalities mainly results from the high sensitivity of FLAIR imaging to subtle changes of longitudinal magnetization in the fluid space, which declines with the higher concentration of the contrast agent, different from T1-weighted images.19,20 The 3D-FLAIR sequence is also known to suppress the signal intensity from the flowing fluid at a velocity exceeding 1.0 cm/s.21 With this background information, we retrospectively evaluated 3D-FLAIR images in 20 subjects with normal facial nerve function and found that only 1 subject showed mild enhancement of the anterior genu segment. From the preliminary study, we hypothesized that the pathologic enhancement of facial neuritis may be more accurately imaged on 3D-FLAIR images than on T1-FFE images, without normal enhancement of the arteriovenous plexus along the facial nerve.
Therefore, the purpose of this study was to investigate the diagnostic performance of CE 3D-FLAIR images compared with CE 3D-T1-FFE images in patients with facial neuritis.
Materials and Methods
This retrospective study was approved by our institutional review board for human investigation, and informed consent was waived.
Patients
We initially selected 55 consecutive patients who presented with facial palsy at our institution between January 2008 and March 2009 and underwent temporal bone MR examination. Of these, 19 patients were excluded for the following reasons: 1) intracranial tumor was detected at symptomatic side on MR imaging (n = 15); 2) patients had CNS infection on clinical evaluation (n = 2); 3) a patient had recent trauma at symptomatic side (n = 1); and 4) a patient had a previous history of temporal bone surgery (n = 1). Thus, 36 patients (mean ± SD age, 50 ± 17 years; range, 1–78 years) were included in our study. The mean ± SD time interval between symptom onset and MR examination was 6 ± 5 days (range, 0–30 days). All 36 patients had been diagnosed with unilateral facial neuritis by physical examination and EPS. MR imaging was performed to exclude a neoplastic cause of facial palsy. Initial House-Brackmann grade22 was II in 6 patients, III in 12, IV in 16, and V in 2.
MR Examination
All MR images were performed with a 3T MR unit (Intera Achieva; Philips Medical Systems, Best, the Netherlands), using an 8-element phased array SENSE head coil. MR images included for image analysis were pre- and postcontrast VISTA-FLAIR sequences and 3D-T1-FFE-fat suppression sequences using Gd-DOTA (Dotarem; Guerbet, Paris, France) at 0.1 mmol/kg of body weight. The order of scanning was uniform in all patients, the 3D-T1-FFE-fat suppression sequence followed by the 3D-FLAIR, with time delays of 3 and 7 minutes, respectively.
The parameters for 3D-FLAIR were as follows: TR, 8000 ms; TE, 268 ms; TI, 2400 ms; modulation of flip angle for refocusing pulses; section thickness, 0.6 mm; overcontiguous sections; 60 sections; FOV, 18 × 18 cm; matrix size, 512 × 512; NEX, 1; SENSE factor, 2; and acquisition time, 6 minutes 6 seconds. The parameters for 3D-T1-FFE-fat suppression were as follows: TR, 25 ms, TE, 4.6 ms; flip angle, 30°; FOV, 18 × 18 cm; matrix size, 512 × 512; section thickness, 0.6 mm; overcontiguous sections; NEX, 1; SENSE factor, 2; and acquisition time, 3 minutes 57 seconds.
MR Image Analysis
Each facial nerve was divided into 5 segments: the canalicular, labyrinthine, anterior genu, tympanic, and mastoid segments. The pattern of enhancement was visually assessed by 2 neuroradiologists for 72 temporal bones (36 each on the left and right sides), with the readers unaware of the lesion side and the prevalence of facial neuritis. After a training interpretation session of 10 cases, selected from 36 patients' data by random sampling, the 2 readers independently reviewed pre- and postenhanced 3D-T1-FFE sequences first, and pre- and postenhanced 3D-FLAIR sequences at 2-week intervals. The 2 datasets for each patient were randomly interpreted during different sessions. For analysis of the same sequence set, we first evaluated the right side of the temporal bone in all patients, and then the left-side images, to minimize the issue of satisfaction of search and tendency of overestimation of the degree of enhancement. Degree of enhancement of each segment was estimated using a 3-point rating scale: 0, no enhancement; 1, mild enhancement, when enhancement was recognized only after comparison with the corresponding precontrast scan; and 2, definite enhancement, when enhancement was definite without comparison.
Statistical Analysis
ROC analysis, using MedCalc for Windows (version 11.2.1; MedCalc Software, Mariakerke, Belgium), was used to evaluate the diagnostic performance of the 2 MR imaging sequences in detecting facial neuritis. For each reader, the AUCs were compared for each dataset using the Z-test.
The sensitivity, specificity, and overall accuracy of each sequence were directly calculated using the criteria of mild (grade 1) or definite (grade 2) enhancement of at least 1 segment of a facial nerve, or enhancement of the canalicular or anterior genu segment only. The results of each sequence were compared using the McNemar test. We also evaluated interobserver agreement between the 2 readers using κ statistics. Pearson χ2 test was used to evaluate the association between the enhancement of each segment and the presence of facial neuritis on CE 3D-FLAIR sequences. In every statistical analysis, a P value <.05 was considered statistically significant.
Results
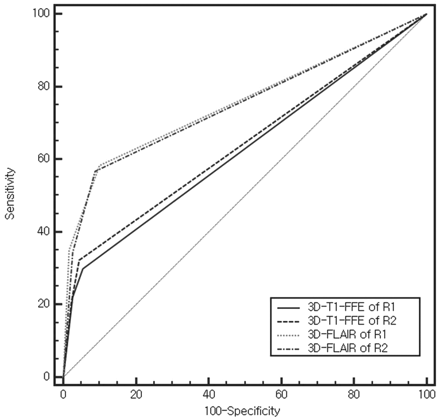
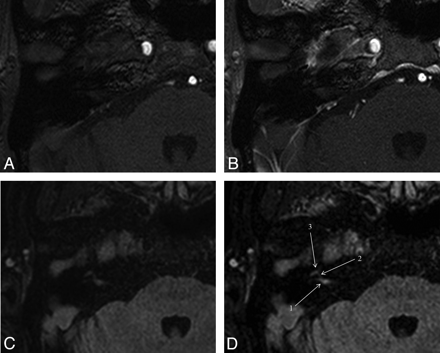
The AUCs of the 2 readers are shown in Fig 1 and Table 1. Figure 2 shows a representative case. From the ROC curves, we found that a score of 1 was the optimal cutoff value for determining the presence of facial neuritis in each segment. Therefore, segments with scores of 1 or 2 were considered positive for facial neuritis.
Receiver operating characteristic curves for diagnosis of facial neuritis. R1 indicates reader 1; R2 = reader 2.
AUC for detection of facial neuritis
MR images of a 61-year-old male with right facial neuritis. On unenhanced (A) and gadolinium-enhanced (B) 3D-T1-FFE images of the right temporal bone, both readers scored 0 (no enhancement) in the canalicular segment. But on unenhanced (C) and gadolinium-enhanced (D) 3D-FLAIR images, both readers scored 2 (definite enhancement) in the canalicular segment. 1 indicates canalicular segment; 2 = labyrinthine segment; 3 = anterior genu segment.
For both readers, the AUCs for the CE 3D-FLAIR were greater than those for the CE 3D-T1-FFE sequence (P < .001). The sensitivity, specificity, and accuracy for detection of facial neuritis were 100% (72/72), 56.9% (41/72), and 78.5% (114/144), respectively, for CE-3D-T1-FFE, and 97.2% (70/72), 86.1% (62/72), and 91.7% (132/144), respectively, for CE 3D-FLAIR. The specificity and accuracy of the CE 3D-FLAIR sequence were significantly better than those for the CE 3D-T1-FFE sequence for detecting facial neuritis (specificity, P = .029; accuracy, P = .008).
Interobserver agreements were excellent for both CE 3D-FLAIR and CE 3D-T1-FFE (κ-value, 0.831 vs. 0.694). Table 2 shows the correlation between the enhancement of each segment of a facial nerve by each reader and the presence of facial neuritis on CE 3D-FLAIR sequences. According to each segment of the facial nerve on CE 3D-FLAIR, we found that enhancement of the canalicular and anterior genu segments were significantly correlated with the presence of facial palsy (P < .001 for canalicular; P = .032 and 0.020 for anterior genu by reader 1 and reader 2, respectively). When we considered the presence of enhancement of the canalicular and anterior genu segments only, the sensitivity, specificity, and accuracy for detection of facial neuritis were 97.2% (70/72), 83.0% (60/72), and 90.2% (130/144), respectively, for CE 3D-T1-FFE, and 95.8% (69/72), 86.1% (62/72), and 90.9% (131/144), respectively, for CE 3D-FLAIR.
Correlation between the enhancement (score 1 or 2) of each segment on CE 3D-FLAIR and presence of facial neuritis
Discussion
In this report, we compared a commonly used CE 3D-T1-FFE sequence with the recently developed CE 3D-FLAIR sequence to assess their ability to evaluate facial neuritis, and we found that the AUC, specificity, and accuracy of CE 3D-FLAIR were greater than those of 3D-T1-FFE. To our knowledge, this is the first report to describe an enhancement pattern of the facial nerve and to evaluate diagnostic performance using CE 3D-FLAIR in patients with facial neuritis.
Enhancement of normal facial nerves on conventional MR imaging is well known and can be explained by the flux of contrast material in the abundant circumneural AVP, which supplies the facial nerve in the facial canal. The vascularity of the AVP is most lush in the geniculate-proximal greater superficial petrosal nerve and in the tympanic and mastoid segments of the facial canal, but this vascularity abruptly terminates at the labyrinthine segment and the stylomastoid foramen.11 Balkany et al,23 in their study on human temporal bones and fresh cadaver nerves, demonstrated that the labyrinthine segment of the facial nerve contains fewer and smaller intrinsic blood vessels than do the mastoid and tympanic segments. This normal enhancement of the facial nerve often hinders the clinical use of conventional MR imaging in clinical practice both for diagnostic purposes and for predicting outcomes despite its high sensitivity.7,24⇓–26
Our results showed that the CE 3D-FLAIR sequence has an advantage in evaluating the pathologic enhancement of the facial nerve over the 3D-T1-FFE sequence, with higher specificity. The results from our study are likely due to the characteristics of the FLAIR sequence. First, this sequence is both more sensitive to lower gadolinium concentrations and less sensitive to higher gadolinium concentrations than T1-weighed sequences. Thus, regions containing higher amounts of gadolinium do not appear enhanced on CE 3D-FLAIR because the signal intensity–reducing T2 effect will obscure the signal intensity–enhancing T1 effect.27 Second, its sensitivity to gadolinium contrast is abruptly reduced as flow velocity increased above 1 cm/s.21 From these characteristics of the FLAIR sequence, the prominent contrast enhancement of the lush circumneural AVP surrounding the normal facial nerve was no longer visible on CE 3D-FLAIR sequences, which resulted in higher sensitivity and overall diagnostic accuracy compared with 3D-T1-FFE sequences. In addition, we used an isotropic 3D imaging technique (VISTA) on a 3T MR system.13 This sequence uses variable refocusing flip angles to constrain T2 decay over a long echo train, with minimal blurring, and can acquire isotropic resolutions in clinically acceptable scan times. Therefore, the disadvantages of FLAIR sequences, including lower SNR and CNR compared with T1WI, using spin-echo or fast-field echo, could be overcome with this 3D acquisition technique, which also enhanced the diagnostic performance of CE 3D-FLAIR over that of CE 3D-T1-FFE.14,15
On the segment-based analysis, we found that enhancement of the canalicular and anterior genu segments were significantly correlated with the presence of facial palsy, but the labyrinthine segment between the canalicular and anterior genu was not correlated with the facial palsy. This may be due to the small size of the fallopian canal and the possible susceptibility artifact from the surrounding petrous bone, which could result in relatively low SNR and CNR in that area.
Our study has several limitations. First, we constantly maintained the order of scanning in which the 3D-T1-FFE and the CE 3D-FLAIR sequences were obtained after contrast injection. The order of pulse sequences can affect the amount of contrast accumulated in the inflamed nerve over time, which, in turn, can result in the particular pulse sequence erroneously appearing to cause more contrast enhancement than that performed earlier in the scan session. However, according to the dynamic study of the 3D FSPGR sequence by Furutani et al28 the 3D FSPGR sequence reaches peak enhancement after 60 minutes, which persists for 6 minutes. We obtained the 3D-T1-FFE followed by the 3D-FLAIR with a time delay of 3 and 7 minutes, respectively. Therefore, there is little chance for the inflamed nerve to be more enhanced on the 3D T1-FFE sequence by switching the order of scanning. Second, the timing from onset of facial palsy to MR imaging ranged from 0–30 days, and this time variability may have affected the degree of enhancement of the affected nerves. Third, we assessed relatively few individuals, and the small sample size may reduce the power of the statistical analysis. Finally, we did not evaluate the clinical significance of the CE 3D-FLAIR sequences, such as their correlation with disease severity, EPS results, and patient prognosis.
Conclusions
Despite some potential limitations, we found that CE 3D-FLAIR sequences were superior to CE 3D-T1-FFE sequences in improving the diagnostic performance of MR imaging, and that 3D-FLAIR is a helpful diagnostic technique in patients with idiopathic facial palsy. Our findings indicated that, following the administration of contrast materials, 3D-FLAIR sequences can be routinely used to evaluate patients with idiopathic facial palsy. Future studies in large patient populations may show whether CE 3D-FLAIR sequences can be used to predict outcomes of acute facial palsy.
References
- Received January 31, 2011.
- Accepted after revision June 29, 2011.
- © 2012 by American Journal of Neuroradiology