Abstract
SUMMARY: MR imaging is the preferred technique for the diagnosis, treatment planning, and monitoring of patients with neoplastic CNS lesions. Conventional MR imaging, with gadolinium-based contrast enhancement, is increasingly combined with advanced, functional MR imaging techniques to offer morphologic, metabolic, and physiologic information. This article provides updated recommendations to neuroradiologists, neuro-oncologists, neurosurgeons, and radiation oncologists on the practical applications of MR imaging of neoplastic CNS lesions in adults, with particular focus on gliomas, based on a review of the clinical trial evidence and personal experiences shared at a recent international meeting of experts in neuroradiology, neuro-oncology, neurosurgery, and radio-oncology.
ABBREVIATIONS:
- ADC
- apparent diffusion coefficient
- CBV
- cerebral blood volume
- CNS
- central nervous system
- DCE
- dynamic contrast-enhanced
- DSC
- dynamic susceptibility contrast
- DTI
- diffusion tensor imaging
- DWI
- diffusion-weighted imaging
- FLAIR
- fluid-attenuated inversion recovery
- FSE
- fast spin-echo
- GFR
- glomerular filtration rate
- GRE
- gradient recalled-echo
- Ktrans
- volume transfer coefficient
- MRS
- MR spectroscopy
- PET
- positron-emission tomography
- PWI
- perfusion-weighted imaging
- rCBV
- relative cerebral blood volume
- TSE
- turbo spin-echo
Neoplastic CNS lesions are a heterogeneous group of diseases with a variable outcome that reflects the precision of diagnosis and the delivery of optimal and specific treatment. CNS imaging has a pivotal role in directing management decisions.
The goals and requirements for CNS imaging are multiple and include the establishment of a diagnosis and differential diagnosis, with accurate lesion grading for characterization of tumor biology. Imaging is an essential part of the decision-making process for therapy and later for planning of surgical or radiotherapeutic interventions. In the case of neurosurgery, neuroimaging can precisely define the location and accurately delineate the lesion and its relationship to eloquent gray- and white-matter structures, before intervention. In radiation therapy, imaging can define and demarcate margins for therapy planning. Imaging is mandatory after therapeutic intervention for monitoring disease and possible side effects.
MR imaging is the standard technique for visualizing and characterizing neoplastic CNS lesions, with superior sensitivity compared with alternative modalities.1⇓⇓–4 Diagnosis and treatment planning are routinely based on conventional MR imaging, such as T2-weighted imaging, FLAIR, and T1 unenhanced FSE or GRE. Following contrast-enhanced T1-weighted imaging, sequences including 3D GRE or 2D TSE or FSE are used routinely for assessment of brain tumors. Through enhancing tissue relaxation, gadolinium-based contrast media improve the sensitivity and specificity of conventional and perfusion-weighted MR imaging examinations, with the capability to identify lesions not visible on unenhanced MR imaging and to provide additional information on lesion morphology, delineation, physiology, and biology.2,5
Gadolinium-based contrast media available for use in MR imaging include the following: gadobenate dimeglumine (MultiHance; Bracco, Milan, Italy), gadobutrol (Gadovist/Gadavist; Bayer Pharma, Berlin, Germany), gadodiamide (Omniscan; GE Healthcare, Chalfont St. Giles, United Kingdom), gadopentetate dimeglumine (Bayer Pharma), gadoterate meglumine (Dotarem; Guerbet, Aulay-sous-Bois, France), gadoteridol (ProHance; Bracco), and gadoversetamide (OptiMark; Mallinckrodt, St. Louis, Missouri). These agents are available as 0.5-mol/L formulations, with the exception of gadobutrol, which is a second-generation agent available as a high-concentration 1.0-molar formulation. All currently available gadolinium-based contrast media demonstrate low (<2%) rates of qualitatively similar adverse drug reactions in surveillance studies.6⇓⇓–9 Clinical trial experience supports these observations and additionally indicates no relationship between gadolinium dose and the incidence of adverse reactions.10
In recent years, a number of advanced, nonenhanced, and contrast-enhanced MR imaging techniques have been developed that not only provide better specificity but offer new insights into the pathophysiology of brain tumors, mainly gliomas. These techniques, including MRS, PWI, DCE MR imaging, and DTI, are increasingly incorporated into imaging protocols and complement the morphologic detail of conventional MR imaging studies, with a range of applications including assessment of treatment response (Table 1).11
CNS applications of advanced MR imaging techniques
The evolution of applications and protocols for MR imaging prompts a continued reassessment of the optimal use of this technique in clinical practice. Furthermore, the recognition that contrast enhancement is sometimes nonspecific and may not always be a true surrogate of tumor extension, grading, and treatment response mandates that new criteria be developed and validated to permit accurate assessment of the efficacy of novel therapies. This article reviews current knowledge on the clinical applications of MR imaging of adult neoplastic CNS lesions—in particular, gliomas—and provides recommendations for best practice, derived from a consensus discussion among 9 experts in neuroradiology, neuro-oncology, neurosurgery, and radio-oncology at a recent international meeting, Improving Patient Management by Optimizing MR Imaging of Neoplastic CNS Lesions, held in Zurich, Switzerland (July 13, 2010).
Applications of MR Imaging in Neoplastic CNS Lesions
Differential Diagnosis
The differential diagnosis of tumoral and pseudotumoral (mainly of inflammatory origin) lesions represents a pivotal step in patient assessment that directs subsequent management decisions. Identifying a tumoral lesion at imaging is followed typically by stereotactic biopsy or surgical resection for histologic confirmation. Not infrequently, inflammatory lesions present as single or multiple focal lesions that can be clinically and/or radiologically indistinguishable from a brain tumor. These represent a diagnostic challenge that may require biopsy for definitive diagnosis, which carries significant morbidity and may itself be nondiagnostic.
Conventional MR imaging, including T1-weighted, T2-weighted, and contrast-enhanced T1-weighted imaging, frequently provides imaging features that permit an accurate differential diagnosis between tumoral and pseudotumoral lesions in >50% of cases. Key imaging features include the following:
The Number, Topography, and Morphology of Lesions.
The presence of an additional nonpseudotumoral lesion associated with a tumorlike lesion, involving the periventricular white matter (some with an ovoid shape with the major axis perpendicular to the ventricular wall), the corpus callosum, or the dorsolateral aspect of the spinal cord, is an important feature, which should support the diagnosis of an inflammatory-demyelinating disease of nontumoral origin, particularly in the appropriate clinical setting (young and middle-aged patients without a history of cancer).
The Intrinsic Lesion Architecture.
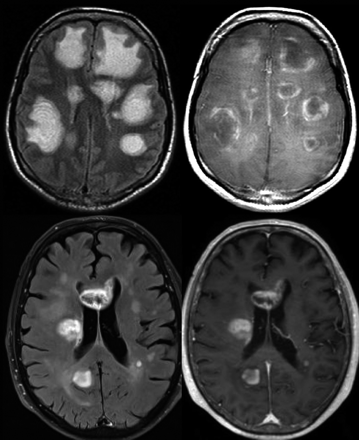
Not infrequently, inflammatory pseudotumoral lesions show a peculiar pattern of concentric or mosaic bands on T2-weighted images, representing alternating layers of preserved and destroyed myelin (Baló-like pattern), which should be considered pathognomonic for these types of lesions (Fig 1).37
Intrinsic lesion architecture. A Baló-like pattern (alternating layers of preserved and destroyed myelin) can be identified in a patient with multiple pseudotumoral inflammatory-demyelinating lesions, which shows peripheral contrast uptake (acute disseminated encephalomyelitis) (upper row). This finding is not typically present in high-grade gliomas, as shown in a patient with multiple hemispheric masses that enhanced after contrast administration, which proved to be a multifocal glioblastoma (lower row).
The Pattern of Contrast Medium Uptake.
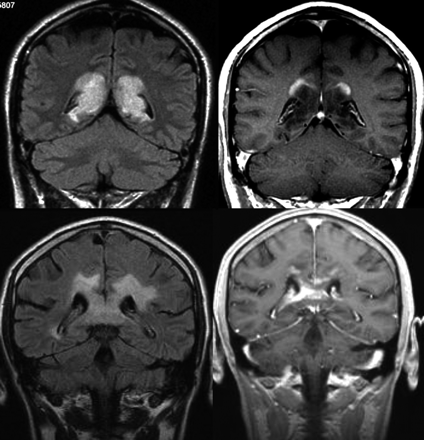
Acute inflammatory pseudotumoral lesions sometimes show an incomplete ring enhancement on T1-weighted gadolinium-enhanced images, with the open border facing the superficial or deep gray matter. This feature, which may be explained by the reduced degree of inflammatory cell infiltration and blood-brain barrier disruption of these lesions when involving the gray matter, is helpful for distinguishing inflammatory-demyelinating lesions from other focal lesions such as high-grade gliomas or metastases, where the ring enhancement surrounding a central area of necrosis is complete, independent of its relation to the gray matter (Fig 2).
Patterns of contrast media uptake. A large tumefactive inflammatory lesion involving the corpus callosum shows an open ring enhancement with the open border facing the cortical gray matter (upper row). This feature is not typically seen in high-grade gliomas, where peripheral enhancement is identified even in the margins of the lesion in contact with the gray matter (lower row).
In addition to these features based on conventional MR imaging, advanced but otherwise routine techniques in daily practice, such as DWI sequences, provide useful information for providing an accurate differential diagnosis between pyogenic abscesses and necrotic tumoral lesions (both metastases and high-grade gliomas), because typically the viscous purulent content of abscesses induces a marked diffusion restriction and, as a consequence, strongly reduced ADC.37 However, this technique cannot distinguish necrotic brain tumors from inflammatory pseudotumoral cystic lesions, because in both cases, DWI may show a rim of decreased diffusion (low signal intensity on ADC maps) surrounding the central necrotic or cystic area with increased diffusion (high signal intensity on ADC maps).
The capability of proton MRS to differentiate pseudotumoral lesions from brain tumors is unresolved in the literature. While some authors report that spectral differences are insufficient to provide a precise diagnosis,38,39 others conclude that discrimination is possible by using a pattern-recognition system.40 Metabolites investigated in these studies include N-acetylaspartate (an index of neural tissue viability), choline (a measure of cell membrane attenuation and turnover), glutamate and glutamine (markers of inflammatory processes), and myo-inositol (indicator of glial cell proliferation). Creatinine is a widely used marker of overall brain metabolism. Saindane et al41 reported significant differences in N-acetylaspartate/creatinine ratios between the central regions of pseudotumoral inflammatory lesions and high-grade gliomas. Cianfoni et al42 observed marked elevations of glutamate and glutamine peaks at short TE in pseudotumoral inflammatory lesions that were not typical of high-grade gliomas. Majos et al43 reported discrimination between brain tumors and pseudotumoral lesions by using myo-inositol/N-acetylaspartate at short TEs and choline/N-acetylaspartate at long TEs. Despite these observations, caution is advised before basing a diagnosis on spectroscopic findings alone.
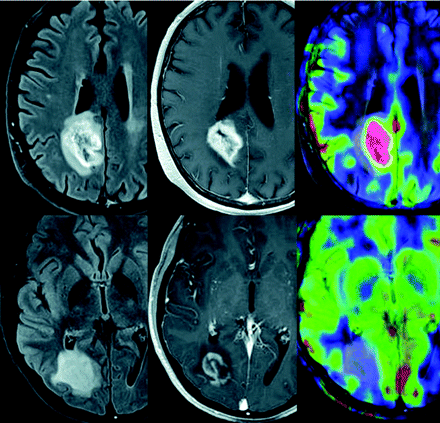
Perfusion MR imaging studies can be helpful in differentiating pseudotumoral inflammatory lesions and high-grade gliomas because only the latter show a significant increase in CBV (Fig 3).44 However, despite the generally decreased cerebral blood flow in inflammatory lesions, a transient increase may be identified in the very early phase of the formation of these lesions.17 A pitfall in perfusion imaging (DSC MR imaging) of lesions is that a very leaky blood-brain barrier can confound CBV measurements and cause lesions with low CBV or perfusion to artifactually demonstrate high perfusion because of the leakiness.
MR imaging (FLAIR, contrast-enhanced T1-weighted, and cerebral blood flow maps acquired with arterial spin-labeling) obtained in patients with a high-grade necrotic glioma (upper row) and an acute inflammatory-demyelinating lesion (lower row). Observe how, despite similar lesion patterns on both T2- and contrast-enhanced T1-weighted sequences, only the high-grade glioma shows a clear increase in cerebral blood flow.
Conventional MR imaging is capable of distinguishing primary brain tumors from metastatic lesions in most cases, on the basis of the number, location, and architecture of lesions. The use of high-concentration contrast media provides optimal lesion visualization for differential diagnosis. In cases of diagnostic uncertainty, PWI or MRS can increase diagnostic confidence. Elevations of CBV in the peritumoral region (representing tumor infiltration/vascularity) and the choline/creatinine ratio (increased cell membrane turnover) indicate high-grade gliomas rather than metastases.29,45
Primary Tumor Biology and Grading
Histologic Grading and Its Limitations.
Histologic classification remains the criterion standard for grading neoplastic CNS lesions, which reflects the clinical outcome in many instances. The updated, 2007 World Health Organization classification of brain tumors provides each lesion type with a name (based on cell type and histologic criteria), grading (aggressiveness), and a code for whether the mass is benign, malignant, or borderline.46
Critics have noted, however, that problems of classification and grading remain, reflecting both the complexity of neoplastic lesions and the challenges of histologic interpretation, such as sampling error and inter- and intraobserver variability.20 Future revisions of the World Health Organization classification will likely incorporate molecular markers and imaging biomarkers to aid classification of glioma biology. As discussed in this article, MR imaging—particularly by using advanced techniques—can provide additional information on lesion grading relevant to outcome.
Role of MR Imaging and Contrast Enhancement in Tumor Grading.
Low- and high-grade gliomas may be differentiated on conventional MR imaging by characteristic differences in the degree of contrast enhancement and the extent of mass effect and cyst formation, which are elevated in higher lesion grades.47 Low- and high-grade gliomas may, however, present atypically on conventional MR imaging; this outcome has led to investigation of advanced MR imaging for grading.48
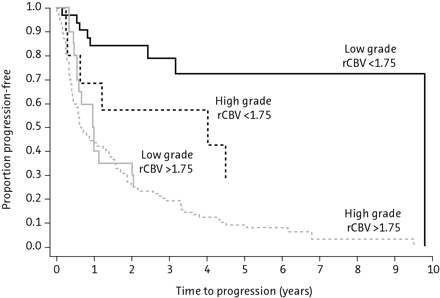
PWI, in particular rCBV measurement, has the capability not only of differentiating low- from high-grade gliomas but of predicting progression and survival.14,19,24,49 In 1 study, a threshold value of 1.75 for rCBV derived from DSC had a sensitivity and specificity of 95.0% and 57.5%, respectively, for differentiating low- and high-grade gliomas, with the ability to predict progression-free survival (Fig 4). With further optimization of acquisition and postprocessing methods, this technique has the potential to be an important biomarker of glioma malignancy and patient outcome.20 Quantification of endothelial permeability by using DCE PWI (most commonly by using Ktrans) is an additional technique with the utility for lesion grading that may be combined with DSC.19
Kaplan-Meier survival curves for progression-free survival within a low-grade glioma group with low and high rCBV (<1.75 and >1.75, respectively; solid lines) demonstrating a significant difference in time to progression in low-grade gliomas stratified by rCBV alone (P < .0001). Similarly, when comparing high-grade gliomas (broken lines), one sees a significant difference in progression with high-versus-low rCBV (<1.75 versus >1.75) (P < .0001). Among subjects with low rCBV (<1.75), there is a significant difference between low- and high-grade gliomas with respect to progression-free survival (P = .047). However, among subjects with high rCBV (> 1.75), progression-free survival is not significantly different for low-versus-high-grade gliomas (P = .266). Reprinted with permission from Radiology (2008;247:490–98). Copyright 2008, Radiological Society of North America.
The use of MRS for glioma grading is promising, particularly when combined with PWI, but the technique remains largely investigational, with drawbacks of low specificity, long acquisition times, and artifacts dependency, particularly in the postoperative phase.14
Role of Contrast Enhancement in Guiding Biopsy.
The histologic composition of lesions can vary markedly, even within the same lesion mass. Biopsy directed by contrast-enhanced imaging, pre- or perioperatively, increases the likelihood of retrieving the most malignant tissue for histologic diagnosis, which will guide treatment decisions.50⇓–52 The use of high-concentration contrast media and advanced MR imaging techniques provides reliable lesion visualization to direct biopsy.53
PWI in this context has proved its utility to locate regions of high vascularity, and hence malignancy, in lesions.19 Diffusion tensor tractography is a promising advanced MR imaging technique for localizing tumor infiltration in 3D and may have applications for intraoperative surgical planning, avoiding eloquent white matter tracts.54
Identification of Tumor Complications
Neurologic complications associated with the lesion itself or its treatment may require emergency assessment and treatment. Clinically important complications include acute ischemic stroke, intracranial hemorrhage, mass effect, hydrocephalus, infection, and spinal cord compression.55 MR imaging is the recognized technique of choice for identifying and directing the treatment of these complications.55
Treatment Planning (Primary Brain Tumors)
Resection.
Surgical resection, commonly followed by postoperative radiation therapy or chemotherapy or both, represents the treatment of choice for high-grade gliomas. A decision to attempt resection is founded on the MR imaging characteristics of tumor size, location, spatial configuration, and presumed margins, together with assessment of the patient's neurologic and medical condition.50⇓–52
Total resection is superior to partial resection or biopsy for providing histologic material and leads to enhanced patient survival.56,57 Total resection overcomes some, but not all, of the limitations of sampling error. Contrast-enhanced MR imaging postoperatively provides assessment of the success of surgery by locating residual tumor.
Radiation Therapy.
The role of radiation therapy remains controversial for low-grade gliomas.58,59 Several groups have evaluated the impact of radiation therapy, showing that radiation immediately after the primary diagnosis can increase progression-free survival; however, overall survival is comparable with that of patients treated for tumor progression of their low-grade gliomas. For glioblastomas, radiation therapy with temozolomide is demonstrated to prolong survival and delay progression and is currently the standard of care.60⇓–62
For treatment of high-grade gliomas, the radiation oncologist defines gross tumor volumes and clinical target volumes, which are based on pre- and postsurgical imaging. A set of unenhanced and contrast-enhanced CT and MR images is generally necessary for radiation therapy treatment planning. For high-grade gliomas, gross tumor volume is commonly defined as the macroscopic tumor visible on T1 contrast-enhanced MR imaging; however, the clinical target volume comprises T2 or FLAIR hyperintense lesions, adding a necessary safety margin to potential microscopic spread. Additional examinations, such as MRS, can provide important information for intricate target-volume definition. As techniques in radiation oncology have evolved, leading to continuously increasing precision of treatment, more detailed and specific imaging examinations are required.63,64Advanced MR imaging techniques postradiation and -chemotherapy have a pivotal role in distinguishing response from pseudoresponse and true disease progression from pseudoprogression. The timing of these scans is critical for accurate assessment, as discussed below.
Non-MR Imaging Modalities: Pros and Cons
MR imaging represents the technique of choice for visualizing and grading brain tumors. However, CT, with a lower resolution than MR imaging, does have applications in the emergency setting, where an MR imaging protocol may be impracticable on the day of presentation. Perfusion CT can provide noninvasive information on tumor vasculature and angiogenesis within gliomas that correlates with histologic and angiographic markers, with relevance to grading and prognosis.65
PET, most commonly with [18F] fluorodeoxyglucose, has a role for directing biopsy, as a prognostic indicator, and for evaluating disease after therapy.35 Radiolabeled amino acids (eg, 11C-methionine, [18F] fluoroethyl-tyrosine) are valuable PET tracers because of their high uptake in tumor tissue.35 Newer tracers are being investigated for the brain, including [18F] fluorothymidine, which may have even higher specificity than glucose-based tracers.53 The sensitivity of PET is enhanced in combination with MR imaging.35
Posttherapeutic Response Assessment
CNS lesions show great variability in their natural course and response to therapy. MR imaging provides an important reference with which to monitor treatment response.
The Macdonald criteria were developed in 1990 for assessing the posttherapeutic response of high-grade gliomas.66 These criteria define disease progression as an increase in enhancing tumor area ≥25% or the appearance of new enhancing lesions. However, the Macdonald criteria have a number of recognized limitations,67 including the following:
Difficulty of measuring irregularly shaped tumors
Interobserver variability
Lack of assessment of nonenhancing lesion components
Lack of guidance for assessment of multifocal tumors
Difficulty of measuring enhancing lesions in the wall of cystic or surgical cavities
After complete resection, no tissue available for assessment.
In addition, increased gadolinium enhancement is a nonspecific finding that may have a number of causes:
A disrupted blood-brain barrier, which can be influenced by changes in steroid dose or radiologic technique68
Nontumoral processes such as treatment-related inflammation, seizure activity, postsurgical changes, ischemia, subacute radiation effects, pseudoprogression, and radiation necrosis.
As a consequence, contrast enhancement may not correlate with changes observed on T2-weighted or FLAIR imaging.67,69 Advanced MR imaging techniques have utility to overcome the limitations of the Macdonald criteria, as reported in the section below.
Consensus Statement
Conventional MR imaging is the technique of choice for differential diagnosis, tumor grading, and treatment planning of neoplastic CNS lesions.
Alternative imaging modalities (CT, PET) have some applications under specific circumstances, frequently as a complement to MR imaging.
MR imaging is an important reference point for monitoring treatment response and recurrence, but the Macdonald criteria have limitations.
Current Limitations of MR Imaging in Neoplastic CNS Lesions
Response Assessment by the Macdonald Criteria
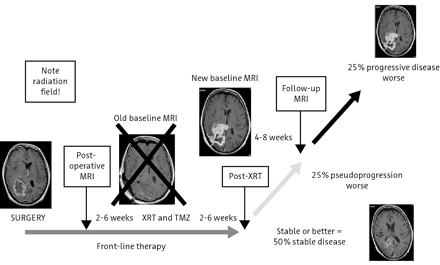
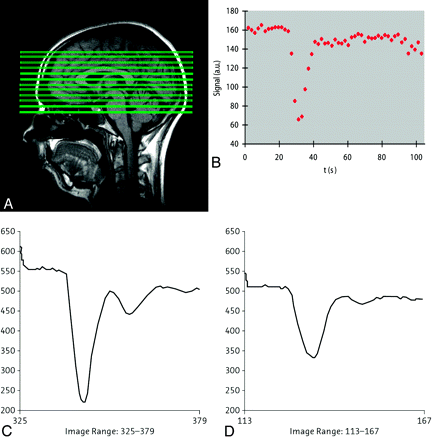
Advances in therapeutic management highlight the limitations of the Macdonald criteria for assessing tumor response. Approximately 10%–30% of patients with glioblastoma treated with the current standard of care—radiation therapy combined with temozolomide—have been proposed to demonstrate “pseudoprogression,” which is defined as increased gadolinium enhancement that does not reflect true tumor progression. Pseudoprogression is most prevalent within the first 12 weeks after completion of radiation therapy and may, if misinterpreted, lead to premature discontinuation of effective therapy (Fig 5).68⇓⇓–71
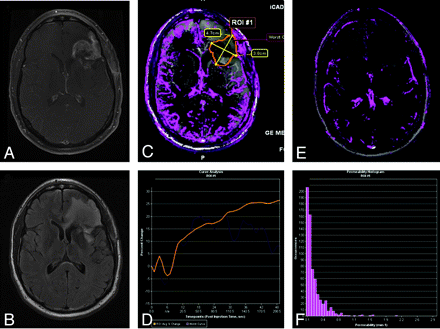
Pseudoprogression in left frontal anaplastic astrocytoma. A, Axial T1-weighted image with contrast shows posttherapeutic brain with nodular contrast enhancement. B, Axial FLAIR image demonstrates increased edema surrounding the enhancing lesion. C, Permeability/Ktrans map with the region of interest. D, DCE MR imaging T1 signal intensity curve demonstrates reduced perfusion and permeability, suggesting pseudoprogression rather than recurrent tumor. Therapy was continued because the findings were thought to be due to pseudoprogression from chemoradiation therapy. E, Permeability/Ktrans color overlay, again confirming decreased vascularity and Ktrans. F, Histogram of each pixel within the region of interest in C, confirming that the permeability is in the lower range, demonstrating pseudoprogression rather than true disease progression. Courtesy of M. Law, Los Angeles, California.
Conversely, antiangiogenic therapies (eg, bevacizumab) can induce a “pseudoresponse” within 1–2 days of initiating treatment, presumably through normalization of the blood-brain barrier.67,69 A subgroup of patients treated with antiangiogenic therapies showed evidence of recurrence, characterized by progressive increases in the nonenhancing component on T2-weighted or FLAIR sequences, which correlate with neurologic deterioration.
New Criteria for Response Assessment
The limitations of the Macdonald criteria, including the recognition that contrast enhancement is nonspecific and may not be a true surrogate of tumor progression or response, have led to the development of new criteria for assessing high-grade gliomas that are summarized in Table 2.67
New criteria for response assessment of high-grade gliomasa
Time course of pseudoprogression and change in the reference or baseline MR imaging. Criteria for determining progression are dependent on the time from initial chemotherapy and radiation. If one takes the reference MR image immediately postoperative, the first 12-week MR image may represent pseudoprogression and pseudoresponse. If one takes the reference scan after that initial 12-week period, then it essentially excludes pseudoprogression. Note enhancement outside the radiation field, where any enhancement may indicate disease progression. To avoid interpretation of postoperative changes as residual enhancing disease, one should ideally obtain a reference MR image within 24–48 hours after surgery and no later than 72 hours after surgery.
Advanced MR imaging techniques, particularly PWI, may assist in differentiating posttreatment changes from the true recurrence of high-grade gliomas.72 Pseudoprogression following radiochemotherapy is characterized by a low rCBV in conjunction with “lowish” permeability (blood-brain barrier leakage) and increased choline, whereas true progression is demonstrated by elevated rCBV (>1.75) and permeability (Fig 4).
Consensus Statement
New criteria for response assessment67 have been developed because of limitations with the Macdonald criteria, offering revised definitions of disease progression.
Appropriate timing of MR imaging reference assessments is critical to the interpretation of response and progression.
A reference MR image should be performed before radiation therapy (preferably within 7 days), with repeat scans at 4 and 12 weeks postradiation.
MR imaging to identify the extent of resection should be performed at latest 72 hours postsurgery.
Contrast enhancement beyond the initial radiation field raises the suspicion of recurrent or progressive tumoral disease, whereas enhancement within the radiation portal could represent pseudoprogression.
Advanced MR imaging with PWI offers advantages for the assessment of pseudoprogression.
Protocols and Techniques for MR Imaging of Neoplastic CNS Lesions
Protocol Sequence and Equipment Optimization
Protocol Sequence.
Optimizing the protocol sequence enhances CNS lesion visualization and characterization. Components of a standardized protocol for conventional MR imaging include T1-weighted precontrast, T2-weighted, DWI, and T1-weighted contrast imaging. FLAIR may be substituted for T2 to save time. PWI, MRS, and other advanced MR imaging techniques may be included in the protocol according to the availability of equipment and the clinical scenario (Table 1). Recommendations for an MR imaging protocol that can be adopted in as many institutions as possible are shown in Table 3.
Standard protocol for brain tumor imaging based on expert panel discussion following the framework of the ACRIN 6686 component of the RTOG 0825 protocol73
Equipment Optimization.
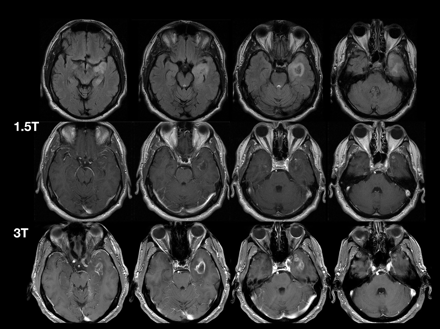
High field strengths provide superior image quality through increased signal intensity. The clinical superiority of 3T over 1.5T has been demonstrated in multiple sclerosis75,76 and, in animal models, in brain tumor imaging.77⇓–79 Case study experience supports superior CNS lesion grading and monitoring with 3T versus 1.5T (Fig 7).
Comparison of MR images at 1.5 and 3T in a patient with astrocytoma grade III after administration of gadobutrol, 0.1 mmol/kg.
Head coils with ≥32 channels, for use with higher field strengths, offer additional signal intensity–to-noise ratio gains compared with conventional equipment, but these coils are currently uncommon in routine practice.80,81 An industry standard at the time of submission is the 8- or 16-channel head coil.
Timing of Image Acquisition.
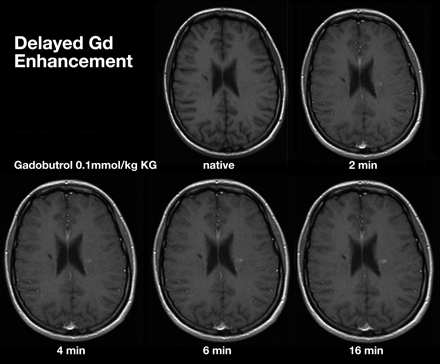
Delays of up to 20 minutes postcontrast injection significantly improve lesion detection rates and the assessment of lesion volume (Fig 8).82,83 Experimental studies by using a rat glioma model suggested that an 8-minute delay postinjection represents a practical compromise between enhanced lesion detection and extended scanning time.84 Delay can be added in by performing DWI and or DTI before acquiring T1 sequences (Table 3).
Comparison of MR images at increasing time intervals after administration of gadobutrol, 0.1 mmol/kg.
Consensus Statement
Optimization of the protocol sequence enhances CNS lesion characterization.
Components of a standardized protocol for conventional MR imaging include T1-weighted precontrast, T2-weighted (or FLAIR), DWI, and T1-weighted contrast imaging.
Incorporation of additional advanced MR imaging techniques (selected according to the clinical scenario) can provide physiologic data to complement morphologic information.
Higher field strengths (eg, 3T) and head coils with multiple channels, if available, are preferred for superior image quality.
Delays of up to 20 minutes postcontrast injection significantly improve lesion detection rates.
New protocol guidelines have been developed for the assessment of therapeutic response67 because of limitations of the Macdonald criteria relating to pseudoprogression and pseudoresponse, especially after chemoradiotherapy with temozolomide and radiation therapy.
Contrast Medium Choice
Chelate Stability.
Gadolinium-based contrast media can be categorized by their molecular structure into linear and macrocyclic groups. Relative to the agents classified in the linear group, media with a macrocyclic structure (gadobutrol, gadoterate dimeglumine, and gadoteridol) demonstrate an increased stability and a reduced propensity to release gadolinium ions in preclinical experiments that include conditions mimicking renal impairment.85,86
The release of gadolinium ions from certain contrast media has been associated with the rare condition of nephrogenic systemic fibrosis in patients with severe renal impairment.87 In separate recent initiatives, the US Food and Drug Administration and the Committee for Medicinal Products for Human Use of the European Medicines Agency have issued guidance on the risk of nephrogenic systemic fibrosis associated with each gadolinium-based contrast medium, placing the macrocyclic agents into lowest-risk groups.88,89
Contrast Enhancement.
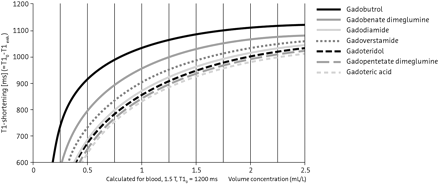
The physicochemical characteristics of contrast media that are associated with the extent of enhancement at MR imaging are their gadolinium concentration and T1 relaxivity. Among current contrast media, gadobutrol possesses the highest gadolinium concentration (1 mol/L) and a high relaxivity, which combine to produce the highest T1 shortening effect per milliliter with the greatest signal intensity (Fig 9).90⇓–92
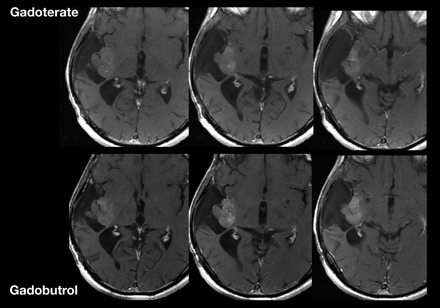
Numerous intraindividual trials have directly compared the imaging characteristics of contrast media in patients with primary CNS lesions or metastases.91,93⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓–104 Among these trials, gadobenate dimeglumine has demonstrated superior lesion enhancement and diagnostic information compared with gadopentetate or gadodiamide,99,100,102 which is attributed to the higher relaxivity of gadobenate. In similarly designed trials, gadobutrol has demonstrated superior performance, including enhanced lesion detection and conspicuity, compared with the 0.5-mol/L agents gadopentetate and gadoterate, administered at the same dose and by using the same field strength (1.5T), reflecting the high T1 shortening effect of gadobutrol (Fig 10).94,98,105
Recurrent right temporoinsular glioma in a 48-year-old patient. Consecutive axial views of T1-weighted images after a single dose (0.1 mmol/kg body weight) of gadoterate dimeglumine or gadobutrol. On gadobutrol-enhanced images, the tumor presents with significantly stronger contrast enhancement, which allows better delineation of suspected anaplastic tumor from nonenhancing tumor areas and adjacent structures.
Animal glioma models support the clinical observations of superior lesion enhancement with gadobutrol versus 0.5-mol/L agents, both at 1.5T and 3T.77,106,107
Direct comparison of gadobutrol at 2 concentrations (with the same total dose) in volunteers demonstrated the benefits of the 1 mol/L over a 0.5-mol/L gadolinium concentration for CNS perfusion imaging, which is attributable to the sharper bolus peak and the increased first-pass gadolinium concentration related to a lower injection volume (Figs 11 and 12).108
A, Sagittal scout MR image with the position of the sections in which perfusion information is acquired. B, Signal intensity–time curve from DSC MR imaging after a bolus injection of a single dose of contrast agent, with substantial signal intensity drop due to the susceptibility effect of the contrast medium. C and D, Signal intensity–time curves from different contrast medium concentrations at a triple dose: 28 mL of the 1.0 mol/L gadobutrol formulation (C) and 56 mL of a 0.5 mol/L gadobutrol formulation (D) in the putamen of the same subject. The susceptibility effect is significantly stronger by using a higher concentration of contrast medium. C and D, reprinted with permission from Radiology (2003;226:880–88). Copyright 2003, Radiological Society of North America.
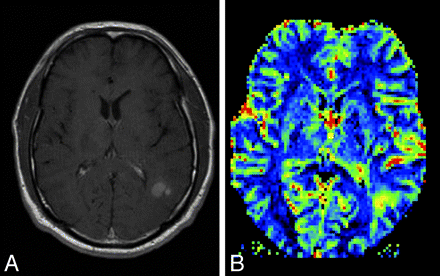
A, Postcontrast T1-weighted MR image in a patient with a new appearance of a contrast-enhancing lesion in a formerly radiotherapeutically treated fibrillary astrocytoma. From conventional imaging sequences, one cannot differentiate treatment-related blood-brain barrier breakdown and malignization of the tumor. B, rCBF perfusion parameter image shows a highly perfused lesion, which was suspicious and later histologically confirmed as a high-grade tumor nodule within the low-grade astrocytoma.
Dosage Recommendations.
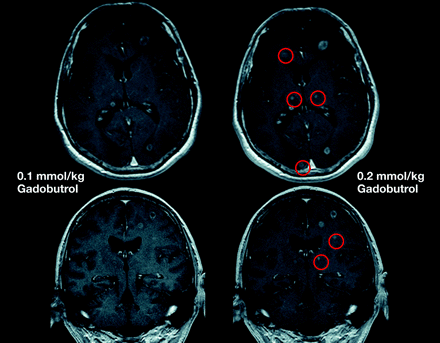
The standard gadolinium dose for contrast media used in MR imaging of the CNS is 0.1 mmol/kg of body weight. Single doses are recommended at many centers for visualization of gliomas, with the option to administer additional doses in cases of diagnostic doubt (Fig 13). For detection of multiple lesions, including metastases, higher concentrations (0.2–0.3 mmol/kg of body weight) may identify additional lesions and are widely used at the initial assessment.4,10,109⇓⇓⇓⇓⇓⇓⇓⇓⇓–119 Double dosing also improves image quality and data quantitation in MR perfusion studies.95
Comparison of MR images by using gadobutrol at 0.1 and 0.2 mmol/kg. Single-dose (left) and double-dose (right) contrast-enhanced MR images in a patient with cerebral metastases. With the use of double-dose gadobutrol, one can detect substantially more lesions (circles) (see also Kim et al 201098) and lesions already visualized with an improved contrast and a better delineation.
Typically, as for all pharmaceuticals, the lowest dose possible should be used. This is particularly important in the context of the risk of nephrogenic systemic fibrosis and especially in patients with severely impaired renal function (GFR below 30 mL/min/1.73 m2).120
Consensus Statement
Contrast enhancement by using gadolinium-based contrast media is a routine component of the MR imaging protocol for patients with CNS lesions.
Chelate stability is an important consideration in the choice of contrast medium, particularly for patients with renal impairment.
Contrast media with high gadolinium concentration and higher relaxivity are preferred for superior enhancement. Gadobutrol (1 mol/L) offers the highest gadolinium concentration and high relaxivity to provide the highest T1 shortening effect among currently available agents.
A single dose (0.1 mmol/kg of body weight) of gadolinium-based contrast medium is recommended for suspected primary lesions, with a second administration in cases of diagnostic doubt.
In patients with a GFR below 30 mL/min/1.73 m2, a single dose only is recommended (with preference for a macrocyclic). With a GFR of 30–60 mL/min/1.73 m2, a single or double dose may be used (preference for a macrocyclic).
Future Challenges
Standardization of an optimized protocol across centers is an important objective, with benefits for the uniform performance and interpretation of MR imaging studies. However, variability among centers in the equipment and the data-interpretation software that are available and a lack of trial evidence to confirm the clinical benefit of novel MR imaging techniques represent barriers to standardized protocol implementation.
Summary of Expert Meeting Recommendations
Recommendation 1: Applications of MR Imaging in Neoplastic CNS Lesions
MR imaging is the technique of choice for the differential diagnosis, tumor grading, and treatment planning of neoplastic CNS lesions.
Advanced MR imaging techniques provide physiologic data relevant to diagnosis and grading that may assist conventional MR imaging.
Recommendation 2: Limitations of MR Imaging—Posttherapeutic Response Assessment
MR imaging is an important reference point for monitoring treatment response and recurrence, but the Macdonald criteria have limitations.
New criteria for assessing enhancing/nonenhancing lesions67 offer amended guidance for response assessment.
Advanced MR imaging techniques may help assess the posttherapeutic brain when contrast enhancement is nonspecific.
Recommendation 3: Standardized Protocol
Optimization of the protocol sequence enhances CNS lesion characterization.
A standardized protocol sequence for conventional MR imaging includes T1-weighted precontrast, T2-weighted, DWI, and T1-weighted contrast imaging. Additional advanced MR imaging techniques can be selected according to the clinical scenario.
Higher field strengths (eg, 3T versus 1.5T) provide superior image quality, if available.
Delay is recommended. Image acquisition at up to 20 minutes postcontrast injection offers improved lesion detection.
Recommendation 4: Contrast Medium Dose
A single dose (0.1 mmol/kg of body weight) of gadolinium-based contrast medium is recommended for suspected primary lesions, with a second administration in cases of diagnostic doubt.
In patients with a GFR below 30 mL/min/1.73 m2, a single dose only is recommended. With a GFR of 30–60 mL/min/1.73 m2, a single or double dose may be used.
Recommendation 5: Contrast Medium Choice
Contrast media with a macrocyclic structure (eg, gadobutrol, gadoterate dimeglumine, and gadoteridol) have a higher chelate stability than linear agents.
Contrast media with high gadolinium concentration and higher relaxivity are preferred for superior enhancement. Gadobutrol offers the highest gadolinium concentration (1 mol/L) and high relaxivity to provide the highest T1 shortening effect among currently available agents.
Footnotes
-
Disclosures: Marco Essig, Consultant: Bayer Healthcare, Details: consultancy agreement. Alex Rovira, Research Support (including provision of equipment or materials): Bayer Schering Pharma; Speaker Bureau: Bayer Schering Pharma, Sanofi-Aventis, Bracco, Merck Serono, Teva Pharmaceutical Industries Ltd, Biogen Idec; Consultant: Novartis. Michael Weller, Speaker Bureau: Bayer Schering, Details: participation in 1 advisory board. Meng Law, Research Support (including provision of equipment or materials): accelerate brain, cancer cure, Details: research grant. Speaker Bureau: Toshiba Medical Systems; Consultant: Bayer Healthcare, Bracco Diagnostics, Fuji Inc, iCAD Inc; Ownership Interest: Prism Clinical Imaging.
-
The expert meeting and the preparation of the review article were funded by an unrestricted educational grant from Bayer HealthCare. PAREXEL provided editorial support.
Indicates open access to non-subscribers at www.ajnr.org
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.
- 13.
- 14.↵
- 15.
- 16.
- 17.↵
- 18.
- 19.↵
- 20.↵
- 21.
- 22.
- 23.
- 24.↵
- 25.
- 26.
- 27.
- 28.
- 29.↵
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.↵
- 36.
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵
- 110.↵
- 111.↵
- 112.↵
- 113.↵
- 114.↵
- 115.↵
- 116.↵
- 117.↵
- 118.↵
- 119.↵
- 120.↵
- © 2012 by American Journal of Neuroradiology