Abstract
BACKGROUND AND PURPOSE: Spinal epidural AVFs are rare spinal vascular malformations. When there is associated intradural venous reflux, they may mimic the more common spinal dural AVFs. Correct diagnosis and localization before conventional angiography is beneficial to facilitate treatment. We hypothesize that first-pass contrast-enhanced MRA can diagnose and localize spinal epidural AVFs with intradural venous reflux and distinguish them from other spinal AVFs.
MATERIALS AND METHODS: Forty-two consecutive patients with a clinical and/or radiologic suspicion of spinal AVF underwent MR imaging, first-pass contrast-enhanced MRA, and DSA at a single institute (2000–2015). MR imaging/MRA and DSA studies were reviewed by 2 independent blinded observers. DSA was used as the reference standard.
RESULTS: On MRA, all 7 spinal epidural AVFs with intradural venous reflux were correctly diagnosed and localized with no interobserver disagreement. The key diagnostic feature was arterialized filling of an epidural venous pouch with a refluxing radicular vein arising from the arterialized epidural venous system.
CONCLUSIONS: First-pass contrast-enhanced MRA is a reliable and useful technique for the initial diagnosis and localization of spinal epidural AVFs with intradural venous reflux and can distinguish these lesions from other spinal AVFs.
ABBREVIATIONS:
- SDAVF
- spinal dural arteriovenous fistula
- SEAVF
- spinal epidural arteriovenous fistula
Spinal epidural or extradural arteriovenous shunting lesions, commonly described as spinal epidural AVFs (SEAVFs), are rare and poorly understood vascular lesions of the spine. These have been described in the literature as case reports or in a few small case series. Their presentation can overlap with that of the more common spinal dural AVFs (SDAVFs) if there is associated intradural venous reflux and congestive myelopathy.1,2 Compared with SDAVFs, the angioarchitecture of SEAVFs with intradural reflux is usually more complex, with the radicular vein arising from the arterialized epidural venous system and with a greater likelihood of multiple arterial feeders and draining veins.1 Diagnosis on noninvasive imaging could alert the angiographer about this. In addition, because the point of reflux into the radicular vein may be at a different level than the arterialized epidural pouch, preangiographic diagnosis may guide DSA for use of appropriate fields of view and delayed runs if required. It could also forewarn the interventional radiologist and/or surgeon to potential greater difficulty in curing these lesions because, in addition to disconnection of the fistula with radicular refluxing vein, obliteration of the arterialized epidural pouch is typically required. In addition, the cross-sectional nature of MRA could depict the involved portion of the epidural venous system in a complementary fashion to DSA, with the ability to view the venous pouch in multiple planes. As such, newer treatments like percutaneous embolization of the epidural venous pouch may benefit from MRA depiction of the lesion.3 The purpose of this study was to evaluate the performance of first-pass contrast-enhanced MRA to diagnose and localize SEAVFs with intradural venous reflux and distinguish them from other spinal AVFs by using DSA as the criterion standard.
Materials and Methods
Study Patients
Approval for this retrospective study was obtained from the local institutional research ethics board of St. Michael's Hospital. Forty-two consecutive patients referred to the St. Michael's Hospital Neurovascular Program with a clinical and/or radiologic suspicion of spinal AVF during the study period (2000–2015) underwent pretherapeutic MR imaging, MRA, and DSA at a single institution. Clinical suspicion was based on typical clinical history of progressive myelopathy and suggestive MR imaging features including nonresolving or progressive cord edema, cord enhancement, and intradural serpentine flow voids and/or enhancing vascularity. Patients with a history of treated spinal AVF were excluded. Between MR imaging, MRA, and DSA studies, patients did not receive any treatment for a spinal AVF.
Note: Some study patients from our data base have been included in another research paper testing a different research question (“First-Pass Contrast-Enhanced MR Angiography in Evaluation of Treated Spinal Arteriovenous Fistulas: Is Catheter Angiography Necessary?” [also in this issue of AJNR]).
MR Imaging and MRA Technique
All patients underwent conventional whole-spine MR imaging on a 1.5T Intera Achieva (Philips, Best, the Netherlands), using a dedicated 5-channel spinal coil with the patient in the supine position, including standard sagittal T2WI, sagittal T1WI, axial T2WI, postcontrast sagittal T1WI, and axial T1WI.
First-pass or bolus-chase contrast-enhanced MRA was performed by using a timed-run technique. The sagittal plane was selected on 3 plane localizers with a field of view of 33 cm (craniocaudal), extending approximately from T3 to L4 vertebral levels. The selection of field of view was based on the clinical/radiologic suspicion of the location of the fistula. After intravenous injection of a 2-mL test bolus of contrast agent, the time taken for filling of the abdominal aorta on MR fluoroscopy was used as delay time for acquisition. Thereafter, 18 mL of contrast agent was administered intravenously at 2 mL/s injection rate by using a 2-cylinder MR compatible injector (Spectris; MedRad, Indianola, Pennsylvania) followed by a 20-mL saline bolus. Studies used gadolinium-based contrast agents, including Omniscan (gadodiamide; GE Healthcare, Piscataway, New Jersey) or, more recently, MultiHance (gadobenate dimeglumine; Bracco Diagnostics, Princeton, New Jersey). Manually triggered, single-phase, 3D acquisition was performed with 400 × 512 matrix and 0.82 × 1.08 mm in-plane resolution reconstructed to 0.64 × 0.64 mm with 0.9-mm section thickness. Scan parameters were: TR = 5.4 ms, TE = 1.76 ms, flip angle = 30°, NEX = 1, overcontiguous sections with scan time of 47 seconds. Automated postprocessing produced background subtracted image sets with multiplanar MIPs.
DSA Technique
Spinal DSA examinations were performed on a dedicated biplanar neuroangiographic system (Artis; Siemens, Erlangen, Germany) via a femoral approach under general or local anesthesia. Multiple selective arterial injections with iodinated contrast agent (Omnipaque 300; GE Healthcare) were performed into the arteries likely to supply the spinal AVF. Magnification, oblique, and high-frame-rate angiography were used where appropriate. The arteries expected to be supplying an AVF based on the MRA findings were catheterized early during the procedure. After identification of the fistula, contralateral injection at the same level and bilateral injections at least 2 vertebral levels above and below the site of the fistula were performed. A complete spinal angiography was undertaken if the AVF was not identified at the anticipated level or MRA was negative for spinal AVF.
Imaging Analysis
Review of MR imaging and MRA studies was independently performed by 2 experienced neuroradiologists (S.P.S. and A.B.) with 13 and 7 years of experience, respectively, without knowledge of the DSA findings or diagnosis.
On the MRA study, the observers made a positive or negative diagnosis of SEAVF with intradural venous reflux and noted the location with regard to vertebral level and side. The presence or absence and location (vertebral level, right/left, ventral/dorsal) of arterialized epidural venous pouch and reflux into the radicular vein as well as presence or absence of additional arterialized epidural veins were noted. The readers had access to source images as well as multiplanar reformats on dedicated workstations.
On conventional MR imaging studies, the presence or absence of intradural serpentine flow voids, T2 hyperintensity of the spinal cord, and cord enhancement were recorded.
Upon completion of review of MR imaging and MRA studies, the observers recorded their findings on DSA studies, including presence or absence of a SEAVF with intradural venous reflux, the location, and key angioarchitectural features of the lesion. The observers again reviewed the conventional MR images to retrospectively identify the arterialized epidural venous pouch. DSA was used as the criterion standard.
Results
There were 31 patients positive for spinal AVFs, of which 7 (23%) were SEAVFs with intradural (radicular/perimedullary) venous reflux. The clinical and MRA findings of these 7 patients are summarized in the On-line Table. The average age of patients was 62 years (range, 54–75 years), and all patients were male. All patients presented with progressive paraparesis. Urinary bladder dysfunction was present in 4 of 7 patients. History of trauma or neurofibromatosis was not recorded for any of the patients. The average duration of symptoms was 4.4 months (range, 0.5–12 months). The average time between MRA and DSA studies was 5 days (range, 0–19 days).
On MRA, the correct diagnosis of SEAVF with intradural venous reflux was successfully made for 7 patients. MRA accurately distinguished SEAVFs with intradural venous reflux from other spinal AVFs (20 SDAVFs, 3 perimedullary spinal AVFs, 1 AVF of filum terminale) (Fig 1) and from the 11 patients with negative DSA with 100% sensitivity, specificity, positive predictive value, and negative predictive value. Lesion localization with respect to vertebral level and location within the spinal canal (right/left, ventral/dorsal) of the epidural venous pouch and connection with the radicular vein was correct in all the cases. However, additional feeders from the contralateral side in 2 cases, with multiple levels in 1 case, that were not identified on MRA were identified on DSA. The radicular vein was correctly detected on MRA as arising from the arterialized epidural pouch (6 cases) or epidural venous system at a different level (1 case). In addition to the epidural venous pouch, vessels conforming to the shape of the epidural venous system were correctly identified to demonstrate arterialized filling in 4 of 7 cases, which matched the catheter angiographic findings. There was no interobserver disagreement. The key diagnostic features of SEAVF distinguishing it from SDAVF were arterialized epidural venous pouch and a refluxing radicular vein from the arterialized epidural pouch or arterialized epidural veins (at a different level) (Figs 2 and 3).
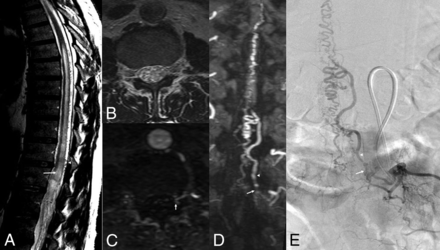
Imaging features of an SDAVF. Sagittal T2WI (A) shows high signal in cord (arrow) and serpiginous flow voids (arrowhead). The ventral epidural space is clear on axial T2WI (B). Axial (C) and coronal (D) reconstructions of MRA-MIP show tuft of vessels at the left L2 dural sleeve (arrow) corresponding to the site of fistula (arrow) on frontal projection on DSA (E). The radicular vein is shown by arrowhead on images D and E.
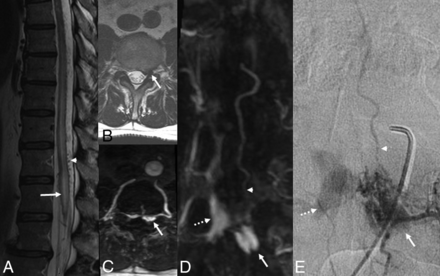
Patient 1. Imaging features of SEAVF with intradural venous reflux. Sagittal T2WI (A) shows high signal in cord (arrow) and serpiginous flow voids (arrowhead). Axial T2WI (B) and axial reconstruction of MRA-MIP (C) show the arterialized left anterolateral epidural venous pouch (arrow). Coronal reconstruction of MRA-MIP (D) and frontal projection on DSA (E) show arterialized contralateral epidural veins (dashed arrow) and the radicular vein (arrowhead) arising from the superior aspect of the venous pouch (arrow).
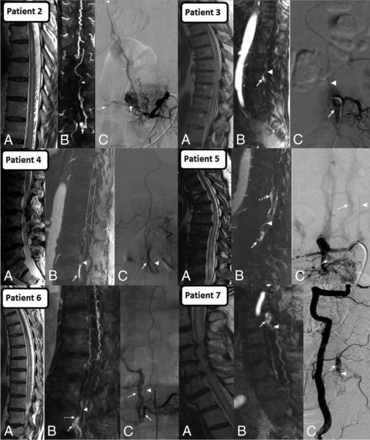
Montage of SEAVFs (patients 2–7). A, Sagittal T2 showing edematous cord with perimedullary flow voids. B, Contrast-enhanced MRA and C, DSA show arterialized epidural venous pouch (arrow), refluxing radicular vein (arrowhead), and additional arterialized epidural veins (dashed arrow). Note that the refluxing radicular vein arises from the arterialized epidural venous pouch except in patient 2, where it arises from the arterialized epidural veins superior to the level of pouch.
All MRA studies in the patient population achieved arterial phase imaging except 1. In this particular case, MRA was motion-degraded with mild venous contamination, which was identified by epidural filling at all scanned levels; no spinal AVF was found on DSA.
On MR imaging, T2 hyperintensity of cord, perimedullary serpentine flow voids, and patchy mild cord enhancement were seen in all 7 (100%) patients. All (24/24, 100%) of the remaining spinal AVFs demonstrated T2 hyperintensity of cord, perimedullary serpentine flow voids, and patchy mild cord enhancement. No significant difference was found in these conventional MR imaging features of SEAVF with intradural reflux compared with other spinal AVFs (P > .05).
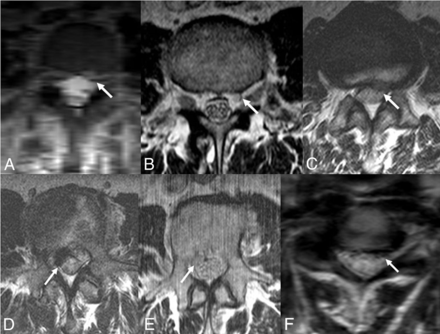
In addition, the epidural arterialized venous pouch seen on MRA studies was retrospectively identified in all cases on conventional MR images. On T2WI, compared with the signal intensity of paraspinal muscles, the pouch was hyperintense in 5 patients and hypointense in 2 patients (Figs 2 and 4). The variable signal and resemblance to disc herniation makes the pouch a subtle finding and difficult to identify without MRA correlation. The MR imaging was negative for epidural hematoma in all the cases.
Epidural venous pouch on T2WI (patients 2–7). Epidural venous pouch (arrow) on axial T2WI. The pouch is a subtle finding with variable signal and simulates disc herniation and, therefore, is hard to identify without MRA correlation.
Discussion
SEAVFs with intradural venous reflux are distinct from the more common SDAVFs. SDAVFs are typically lesions with a single site of fistula at the dural sleeve covering the nerve root, commonly with a single radiculomeningeal artery feeder and draining intradural radicular vein refluxing into the perimedullary venous system. Perimedullary and filum terminale fistulas are variants in which the connection is intradural between radiculopial or radiculomedullary artery and intradural vein, without proximal filling of the epidural system. In SEAVF, which is another variant, the fistula is located in the epidural space and may be associated with 1 or multiple feeding arteries and drain into the epidural venous system.1 These can be asymptomatic because of an antireflux mechanism at the dural sleeve related to inherent narrowing and a zigzag course of the radicular vein while crossing the dura.4 Failure of the antireflux mechanism and/or increased venous pressures due to thrombosis of other outflow veins can result in reflux into radicular and perimedullary veins and venous congestion of the cord causing myelopathy.2 Intradural reflux from the epidural venous system can occur far from the epidural fistula site, which was seen in 1 case in our study and has been described previously.5 Multiple feeders were seen less frequently in our study (2 of 7 cases) compared with previous reports.1,6 Another way these lesions can manifest is by radiculopathy or myelopathy due to direct compression of nerve roots or cord by enlarged epidural veins;7,8 however, this presentation is not discussed in our paper, which focuses on patients who present with congestive myelopathy simulating SDAVF. Because of the difference in angioarchitecture of SEAVFs with intradural venous reflux compared with SDAVFs, the approach to treating these lesions can be different and may be more challenging. Both surgery and endovascular embolization have been described, and a combination of these techniques has also been used.1,5,6,9 Percutaneous embolization of the epidural venous pouch has also been described.3 Pretherapeutic localization and diagnosis through the use of noninvasive imaging may be helpful in expediting subsequent DSA and for planning treatment.
It is unclear if SEAVFs are congenital or acquired; however, association with previous surgery and trauma10,11 as well as neurofibromatosis12,13 has been reported. None of the patients in our study had any such clinical history. In a study by van Rooij et al,14 6% of the spinal AVFs in the study cohort were found to be SEAVFs, compared with 23% found in our study. A literature review of 45 ventral SEAVFs performed by Kiyosue et al6 found that SEAVFs are more common among older males (M:F ratio, 2.4:1; average age, 63.9 years) in the lumbar spine, with progressive myelopathy being the most common symptom, which is similar to the results in our study. In their review, the average duration of symptoms was 10 months,6 greater than our study (4.4 months).
The clinical and conventional MR imaging findings (cord congestion, enhancement, and serpentine flow voids) in SDAVFs and SEAVFs with intradural reflux are similar1,2 because of the similar underlying pathomechanism of venous congestive myelopathy present in both conditions. However, our study indicates that MRA can reliably distinguish these lesions. The key diagnostic features of SEAVFs on MRA are the presence of an arterialized epidural venous pouch and/or filling of the epidural venous system and a refluxing radicular vein. Conversely, in SDAVF, there is a tuft of vessels at the dural sleeve leading to the refluxing radicular vein without arterialized epidural pouch/veins (Fig 1). Although the arterialized epidural venous pouch in SEAVFs could be seen in retrospect on conventional MR images in our study, it was a subtle finding that would be difficult to pick up prospectively. The signal characteristics of the venous pouch on MR imaging were variable, likely because of variable or turbulent flow, adding to the difficulty in identification.
Many studies have proved the utility of MRA for evaluation of SDAVFs, which facilitates the subsequent conventional angiography.15⇓–17 Rangel-Castilla et al18 found DynaCT (Siemens) useful in evaluation of SEAVFs and proposed a novel classification system for these lesions. To the best of our knowledge, our study is the first to evaluate the diagnostic performance of MRA in pretherapeutic noninvasive evaluation of SEAVF with intradural reflux. Our findings suggest high accuracy and reliability of MRA for detection and localization of these lesions and distinguishing them from SDAVFs. This may assist in treatment planning. In 2 cases, additional feeders were identified with DSA, which highlights the limitation of MRA in delineating small vessels.
Limitations of our study include its retrospective design causing selection bias and the small number of patients because of the rarity of this condition. The study was focused toward diagnosis and localization of the lesions and did not attempt to completely characterize the angioarchitecture on MRA, for which DSA remains mandatory. The MRA technique used in the study is a single-phase first-pass MRA technique rather than multiphase time-resolved MRA. This is not a major limitation because although the time-resolved techniques may provide more temporal information, they are limited by lower spatial resolution; hence, we prefer to use first-pass contrast-enhanced MRA. In addition, we have been able to obtain reasonably consistent arterial phase imaging by using this technique at our institute.
Conclusions
First-pass contrast-enhanced MRA is a reliable and useful technique for the initial diagnosis and localization of spinal epidural AVFs with intradural venous reflux and can distinguish them from spinal dural AVFs.
Footnotes
Paper previously presented, in part, at: Annual Meeting of the American Society of Neuroradiology, May 23–26, 2016; Washington, DC.
References
- Received June 13, 2016.
- Accepted after revision August 16, 2016.
- © 2017 by American Journal of Neuroradiology