Abstract
BACKGROUND AND PURPOSE: Cervical spine axial MRI T2-hyperintense fluid signal of the anterior median fissure and round hyperintense foci resembling either the central canal or base of the anterior median fissure are associated with a craniocaudad sagittal line, also simulating the central canal. On the basis of empiric observation, we hypothesized that hyperintense foci, the anterior median fissure, and the sagittal line are seen more frequently in patients with Chiari malformation type I, and the sagittal line may be the base of the anterior median fissure in some patients.
MATERIALS AND METHODS: Saggital line incidence and the incidence/frequency of hyperintense foci and anterior median fissure in 25 patients with Chiari I malformation and 25 contemporaneous age-matched controls were recorded in this prospective exploratory study as either combined (hyperintense foci+anterior median fissure in the same patient), connected (anterior median fissure extending to and appearing to be connected with hyperintense foci), or alone as hyperintense foci or an anterior median fissure. Hyperintense foci and anterior median fissure/patient, hyperintense foci/anterior median fissure ratios, and anterior median fissure extending to and appearing to be connected with hyperintense foci were compared in all, in hyperintense foci+anterior median fissure in the same patient, and in anterior median fissure extending to and appearing to be connected with hyperintense foci in patients with Chiari I malformation and controls.
RESULTS: Increased sagittal line incidence (56%), hyperintense foci (8.5/patient), and anterior median fissure (4.0/patient) frequency were identified in patients with Chiari I malformation versus controls (28%, 3.9/patient, and 2.7/patient, respectively). Increased anterior median fissure/patient, decreasing hyperintense foci/anterior median fissure ratio, and increasing anterior median fissure extending to and appearing to be connected with hyperintense foci/patient were identified in Chiari subgroups. A 21%–58% increase in observed anterior median fissure extending to and appearing connected to hyperintense foci in the entire cohort and multiple sagittal line subgroups compared with predicted occurred.
CONCLUSIONS: In addition to the anticipated increased incidence/frequency of sagittal line and hyperintense foci in patients with Chiari I malformation, an increased incidence and frequency of anterior median fissure and anterior median fissure extending to and appearing to be connected with hyperintense foci/patient were identified. We believe an anterior median fissure may contribute to a saggital line appearance in some patients with Chiari I malformation. While thin saggital line channels are usually ascribed to the central canal, we believe some may be due to the base of the anterior median fissure, created by pulsatile CSF hydrodynamics.
ABBREVIATIONS:
- AMF
- anterior median fissure
- AMF>HIF
- anterior median fissure extending to and appearing to be connected with hyperintense foci
- CTM
- CT myelography
- HIF
- hyperintense foci
- HIF+AMF
- hyperintense foci and anterior median fissure in the same patient
- pt.
- patient
- SL
- sagittal line
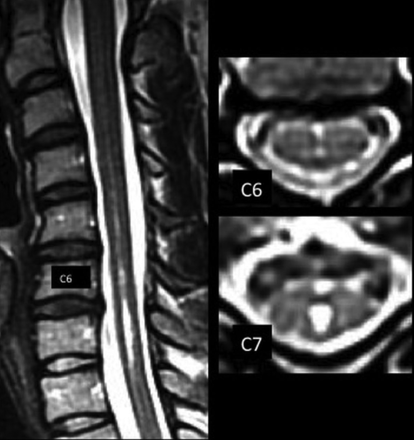
Axial MR imaging of the cervical spine frequently demonstrates hyperintense, linear, anatomically, sagittally-oriented T2 fluid signal of the anterior median fissure (AMF) and hyperintense foci (HIF) resembling the central canal or the base of the AMF.1-3 These axial T2 findings may be associated with a channel-like T2-hyperintense craniocaudad line on images parallel to the sagittal plane (a sagittal line [SL]), simulating the central canal (Fig 1).4,5 A previous analysis of HIF, AMF, and a thin SL in a population without Chiari I malformation provided not only a baseline for their identification but also a confirmation of a relationship between not only the AMF and HIF but also their relationship to the SL.1 It found the following:
HIF were greater in number than AMFs, but AMFs increase in the presence of increasing HIF, suggesting an anatomic relationship.
SLs were associated with greater numbers of both HIF and AMF/patient (pt.) versus no SL, 6.7 versus 2.7/pt. and 3.3 versus 2.0/pt., respectively. SL presence correlated more closely to HIF than to AMF presence within the entire 358-patient group.
When HIF and AMF were classified as combined (concurrent HIF and AMF, with ≥1 of each both present in the same patient [HIF+AMF]) or continuous (AMF appearing to extend to and join an HIF [AMF>HIF]), HIF and AMF/pt. each differed numerically and patients with an SL had more combined HIF+AMF and continuous AMF>HIF than patients without an SL.
In patients with both SL and combined HIF+AMF (a circumstance allowing the possibility of a relationship of all 3 structures), HIF become proportionally fewer compared with AMFs. In patients with an SL actually exhibiting continuous AMF>HIF, the HIF/AMF ratio decreased further.
A patient with Chiari I with 19 HIF up to 3 mm in diameter, 1 AMF, no AMF>HIF, and an SL of various hyperintensity and diameter from C4 through T1, consistent with hydromyelia.
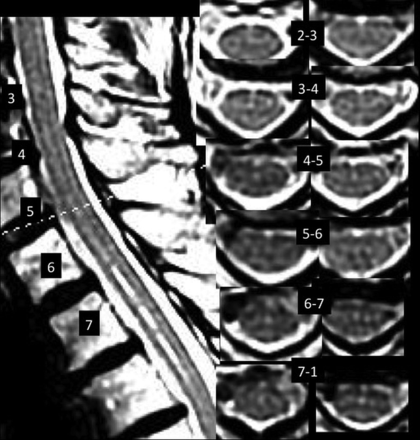
While it is expected that manifestations of the central canal as an SL and HIF are more frequent in patients with Chiari syndrome type I,6 past experience leads us to hypothesize that AMFs are also seen more frequently in patients with Chiari I malformation and that the SL or channel may represent the base of a wide AMF, rather than the central canal, in some patients (Figs 1 and 2). Therefore, we performed an exploratory prospective analysis of HIF, AMF, and SL in patients with Chiari I malformation to examine their relationships.
Postdecompressive craniectomy patient with Chiari I with 9 HIF, 4 AMFs, 1 AMF>HIF, and sharp and hyperintense SLs at C6–C7 and less hyperintense, sharp, and defined SLs at C2–C6.
MATERIALS AND METHODS
The presence of HIF, AMF, and SL on cervical MR imaging in 25 patients with Chiari I malformation and 25 age-matched controls performed on a single scanner was recorded by a single reader. Reader blinding by hiding the tonsil position and postoperative changes was not performed. SL presence was assessed and recorded before HIF and AMF assessment.
The scanning facility is associated with the Mayfield Clinic Chiari Center, a multidisciplinary outpatient center for evaluation and treatment of Chiari I malformation. Patients with Chiari I were symptomatic with headache, dizziness, and/or nystagmus and were confirmed to have a cerebellar tonsil position below the foramen magnum with reduced CSF signal ventral to the cervicomedullary junction at the tectorial membrane and dorsal to the tonsils at the opisthion. The population with Chiari I included 10 postoperative patients. Preoperative images were not available for review. Approval was obtained by Mayfield Clinic and University of Cincinnati institution’s review board.
Technical imaging scanning parameters included the following: a Signa Excite high-definition 1.5T scanner (GE Healthcare); 7.9T/m, 120 T/m/s gradients, standard fast spin-echo T2 sequences; sagittal: 256 × 256 pixel matrix, 3.0-mm thickness, with 1-mm spacing, 24 × 24 cm FOV; axial: 256 × 224 pixel, 3.0-mm thickness, 0.5-mm spacing, 22 × 22 cm FOV. Images were reviewed at ×2.5 magnification on a diagnostic eFilm Workstation (IBM). Patients with an SL diameter of >3 mm, indicative of syringohydromyelia, were excluded from analysis.
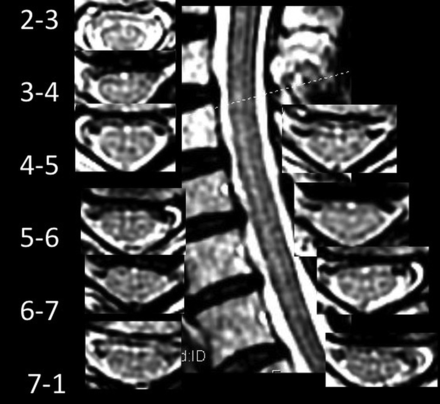
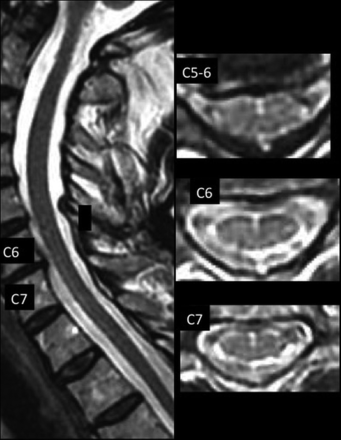
AMFs were recorded as anterior, midline, linear hyperintensities continuing from a dimple-shaped indentation between the ventral hemicords. AMFs may be oriented minimally to the perpendicular.1 HIF were recorded as focal, round, midline fluid-signal hyperintensities of the anterior 25%–50% of the cord. SLs were recorded as sharply-marginated hyperintense craniocaudad lines on sagittal images located in the anterior 25%–50% of the sagittal dimension of the cord (Figs 1 and 2) or as discontinuous craniocaudad alignments of dots and dashes of varying lengths and hyperintensity (Fig 3). They may be associated with wider less hyperintense and less sharp bands and may be seen with partial volume structures (Fig 4).
Control patient with 15 HIF, 3 AMFs, 3 AMF>HIF, and SL with variably hyperintense and variably sharp dots and dashes from C3–4 to C7. Note hyperintense pixels immediately anterior to the AMF on axial images, despite crescentic flow-related signal loss immediately anterior to the cord on most images.
Postoperative patient with Chiari I with 19 HIF, 3 AMFs, and 2 AMF>HIF, with a faintly hyperintense ventral line at C6–T1.
HIF and AMF numbers per patient (frequency) in the control and Chiari I groups were determined. Differences in the incidence of HIF, AMF, SL, and HIF and AMF/pt. between patients with Chiari I and controls were analyzed in Excel (Microsoft) with χ2 and Student t tests. Comparison of the incidence and numbers of AMF+HIF and AMF>HIF/pt. in patients with and without an SL in the Chiari I versus non-Chiari I groups was also performed. Incidence and frequency of HIF and AMF in the 25-patient control group were compared with those of the original, published 356-patient control group.1
RESULTS
Age, sex, and incidence and frequency of HIF, AMF, and SL are listed in Table 1 for the 25 Chiari I, 25 current control, and 356 prior non-Chiari I groups. A female predominance was present in both current groups, with no age difference. No difference was identified in HIF and AMF incidence in the Chiari I and current control groups, present in 88%–100% of both. Both HIF/pt. and AMF/pt. were significantly greater in the Chiari I group compared with current controls (8.5 versus 3.9 HIF/pt. and 4.1 versus 2.7 AMF/pt., respectively). HIF/pt. and AMF/pt. were similar for the current control and the original earlier multiscanner non-Chiari I analysis group1 (3.9 versus 3.7 and 2.7 versus 2.3, respectively), as was the SL incidence (28% versus 25%).
Age, sex, incidence (%), and frequency (No./Pt), for HIF, AMF, SL, and AMF>HIF in the Chiari I and non-Chiari I control groups
No differences in HIF or AMF were identified in 15 patients without an operation versus 10 postoperative patients with Chiari I (Table 2). Twenty HIF measured < 1 mm in width (8 postoperative), 4 measured 1–2 mm (2 postoperative), and 1 patient without an operation had HIF that measured 2–3 mm. (Fig 1). The postoperative group exhibited a higher incidence of SLs (8/10, 80% versus 5/15 [33.3%]), with numerically greater AMF>HIF (2.8 versus 1.9).
Incidence HIF and AMF/pt., SL incidence, and number of AMFs extending to HIF (AMF>HIF) in Chiari I without an operation versus patients having undergone postdecompressive craniectomy
Table 3 outlines HIF and AMF/pt., HIF/AMF ratios, and AMF>HIF/pt. in Chiari I versus controls, with and without SLs. Fourteen patients with Chiari I and an SL demonstrated increased HIF/pt. (10.1 versus 6.6, P < .04) and numerically greater AMF (4.6 versus 3.5, P = .18) versus 11 patients with Chiari I and no SL. Seven control patients with an SL demonstrated increased HIF/pt. (7.0 versus 2.7/pt., P < .04) and greater AMF/pt. (3.4 versus 2.4, P = .02) versus 18 controls without an SL. One patient with Chiari I and HIF-only with 7 HIF and no SL and 1 control with HIF-only with 12 HIF with an SL were identified. One control had no HIF or AMF.
Number patients, HIF/pt., AMF/pt., HIF to AMF ratio, and AMF>HIF/pt. in HIF or AMF-only, HIF+AMF, and AMF>HIF subgroups for all Chiari I and control groups, with and without SL
The observed AMF>HIF/pt. was greater for all patients with Chiari I versus all controls (Online Supplemental Data; 2.2 versus 1.0/pt., P < .001), for 14 patients with Chiari I and an SL versus 7 controls with an SL (2.9 versus 1.3/pt., P < .02), and for 11 patients with Chiari I versus 18 controls without an SL (1.9 versus 0.8, P = .01). The incidence of observed AMF>HIF was also greater than predicted for 12 patients with Chiari I and an SL compared with 5 controls (3 versus 1.8/pt., P < .02) (Online Supplemental Data)
DISCUSSION
Patients with Chiari I are known to have a high incidence of hydrosyringomyelia. Therefore, identification of round foci on axial images (HIF) and thin craniocaudad channels on sagittal images (SL), both usually considered manifestations of the central canal, is anticipated. However, the higher incidence of identification and frequency of an AMF in patients with Chiari I compared with controls has not been previously reported. AMF/pt. increased progressively from all (2.7/pt.), to HIF+AMF (4.3/pt.), to AMF>HIF (4.7/pt.), and to SL AMF>HIF in patients with Chiari I (5.1/pt.). The ratio of HIF/AMF decreased among patients with SL, while AMF/pt. and AMF>HIF/pt. were increasing. AMF>HIF/pt. was highest among patients with an SL (2.9/pt.). Increasing AMF/pt., a decreasing HIF/AMF ratio, and increased AMF>HIF/pt. in patients with SL represent a stronger association of AMF-to-SL identification than suggested in the original non-Chiari I study,1 in which the AMF>HIF/pt. were not counted.
It may be argued that with increases in AMF, identification of an AMF>HIF becomes more likely. However, the observed AMF>HIF/pt. was 47% greater in number than expected on the basis of HIF and AMF numbers for all patients with Chiari I and 58% greater for patients with an SL with AMF>HIF. The correlation of increasing AMF to AMF>HIF/pt. numbers in the setting of a decreasing HIF/AMF ratio supports an etiologic relationship between the visible cleft of an AMF and the round HIF of a potential channel or SL and indicates a potential anatomic connection of the 3 structures. This raises the question of whether some thin craniocaudad channels simulating the central canal on midline sagittal imaging are actually due to widened bases of AMFs. While numbers of AMF>HIF/pt. were not counted in the original non-Chiari I study,1 other measures of HIF, AMF, and SL occurrence in that study are generally consonant with current control findings.
Despite an inability to resolve and separate without question the base of the AMF and HIF or the central canal individually on the same axial image in routine clinical scanning,7 the relationships in our previously published analysis of HIF, AMF, and thin SL in a non-Chiari I MR image population provided not only a baseline control for their incidence of identification but also a confirmation of a relationship among AMF, HIF, and SL.1 This confirmation led us to believe that depictions of HIF and AMF are not totally independent occurrences of 2 different structures (the AMF and the central canal) but rather identification of different manifestations of the same structure: the sagittal cleft of the AMF as well as its wider base, in some instances.
In our prior review of 35 patients without Chiari I with both cervical MR imaging and cervical CT myelography (CTM), iodinated contrast media–filled AMFs were more common than HIF on CTM.1 When present, HIF on CTM appeared almost exclusively as a focal dilation of the base of an AMF on CTM. A number of AMFs on CTM were seen at the same spinal level where MR imaging HIF were identified when an MR imaging AMF had not been identified. In several instances, the AMF on CTM was wide, possibly freely communicating on multiple, contiguous, consecutive axial images, while seen as an atypical broad, indistinct channel on a sagittal image due to partial volume effects on MR imaging. These CTM data are also consistent with HIF representing a wide base of the AMF.8,9
Our findings raise the question of the physiologic origin and basis for imaging findings of the increased HIF, AMF-related channel, or SL simulating hydromyelia on MR imaging. CSF flow dynamics are known to be altered in patients with Chiari I, resulting in imaging of complex, pulsatile CSF motion that is subject to greater or lesser bright-T2 conspicuity with certain pulse sequences, even varying between systole and diastole.10⇓-12 Pulsatile CSF flow effects directed in a Craniodaudal or transverse radial fashion that have been suggested to cause syringomelia13 may be transmitted to the AMF, whose entire cervicomedullary course may not be subject to equal directions and magnitude of force. This possibility may be suggested by variable signal changes of flow and flow-related signal loss on axial images (Figs 3 and 4). Pial/arachnoidal coverings and vessels within the AMF14 may limit communication with the subarachnoid space and secondarily alter local transverse pressure transmission, but they may not limit and may even amplify craniocaudad effects (Figs 1–4). The effects may vary among subjects, and their depiction may vary among field strengths and imaging parameters applied. A formula for the production and/or loss of T2-signal in the AMF has yet to be elucidated.
The main limitation of this study lies in subjective observations of a single, potentially biased reader. That the current explorative study identified control SL incidence similar to prior non-Chiari study (28% versus 25%) as well as similar AMFs and HIF/pt. (3.9 versus 3.7 HIF, and 2.7 versus 2.3 AMF/pt.) suggests that scanners and reader performed similarly in this regard. However, the higher incidence of both HIF and AMF in 25 controls was seen compared with the original 6-scanner retrospective control study with 2 readers (96% versus 66% HIF, 88% versus 60% AMF). Scanner and reader performance may explain this discrepancy. Wide ranges of contributions to the identified HIF and AMF totals were found in the original 2-reader, 6-scanner analysis of controls without Chiari I: 5.9%–15.4% for HIF and 5.9%–16.7% for AMF among the 6 individual scanners (Online Supplemental Data).1 At these rates of identification, a hypothetic similar top-performing scanner might record nearly 100% HIF and/or AMF (16% × 6 scanners = 96%) in a Chiari I population, and 6 similar lowest-performing scanners, just 36% (6% × 6 = 36%). The single scanner of the current study did perform at a similar level for HIF and AMF incidence compared with the highest-performing scanner in the prior 6-scanner non-Chiari I study. However, no single scanner performed best for identification of HIF, AMF, and SL, and no single scanner performed worst for individual structures in the prior study.
That the percentage of SLs was similar for both control subgroups without Chiari I is also consistent with the anticipated range of scanner performance (identified as 12.7%–34.2% in the 6-scanner study) and with the κ analysis in the original study, in which both readers agreed that <70% of patients did not have an SL. The small numeric increase of SLs in patients with Chiari I without operations versus controls is consistent with the original study, in which scanners that identified high numbers of HIF and AMFs did not identify high numbers of SLs. The discrepancy may reflect case selection, in which many patients with Chiari I with larger channels were already excluded from analysis. That a higher percentage of SLs was identified in postoperative patients with Chiari I may indicate a bias in favor of an operation for patients with hydrosyringomyelia preoperatively. The numeric increase of AMF and AMF>HIF in patients with Chiari with and without an operation remains of interest. A more comprehensive review of a larger population of pre- and postoperative scans in patients with Chiari I is required to answer these questions.
The observations reported here may appear to be of little immediate and direct clinical importance, because thin channels of <3 mm in patients with Chiari I are thought to be of little clinical consequence.5,15 However, MR imaging reports attributing an SL or thin channel in a patient with Chiari I to hydro- or syringomyelia may increase patient concern, causing him or her to consider any neurologic symptom as potentially related, leading to unnecessary restudy. To solidify a relationship between conspicuous AMFs and such SLs or channels would serve to diminish concerns based on historical hydrosyringomyelic relationships.
CONCLUSIONS
We identified an increase of not only HIF and SLs in patients with Chiari I compared with controls as anticipated, but also of AMFs, not previously reported. The presence of increased frequency of HIF, AMFs, and thin SLs in the Chiari I population is consistent with a hydrodynamic effect likely created by CSF pressure/pulsation/flow phenomena. We believe that HIF and AMF are also manifestations of the same structure in some instances and may be responsible for some thin SL channels seen in some healthy patients and those with Chiari I as well, not due to the central canal or hydromyelia but rather to the widened base of the AMF.
Footnotes
Paper previously presented, in part, at: Annual Meeting of the American Society of Neuroradiology and Foundation of the ASNR Symposium, April 22–27, 2017; Long Beach, California.
Disclosures: Mario Zuccarello—UNRELATED: Employment: University of Cincinnati.
References
- Received June 26, 2021.
- Accepted after revision November 6, 2021.
- © 2021 by American Journal of Neuroradiology