Abstract
BACKGROUND: Radial artery access for cerebral angiography is traditionally performed in the wrist. Distal transradial access in the anatomic snuffbox is an alternative with several advantages.
PURPOSE: Our aim was to review the safety and efficacy of distal transradial access for diagnostic cerebral angiography and neurointerventions.
DATA SOURCES: We performed a comprehensive search of the literature using PubMed, Scopus, and EMBASE.
STUDY SELECTION: The study included all case series of at least 10 patients describing outcomes associated with distal transradial access for diagnostic cerebral angiography or a neurointervention.
DATA ANALYSIS: Random-effects models were used to obtain pooled rates of procedural success and complications.
DATA SYNTHESIS: A total of 7 studies comprising 348 (75.8%) diagnostic cerebral angiograms and 111 (24.2%) interventions met the inclusion criteria. The pooled success rate was 95% (95% CI, 91%–98%; I2 = 74.33). The pooled minor complication rate was 2% (95% CI, 1%–4%; I2 = 0. No major complications were reported. For diagnostic procedures, the combined mean fluoroscopy time was 13.53 [SD, 8.82] minutes and the mean contrast dose was 74.9 [SD, 35.6] mL.
LIMITATIONS: A small number of studies met the inclusion criteria, all of them were retrospective, and none compared outcomes with proximal transradial or femoral access.
CONCLUSIONS: Early experience with distal transradial access suggests that it is a safe and effective alternative to proximal radial and femoral access for performing diagnostic cerebral angiography and interventions. Additional studies are needed to establish its efficacy and compare it with other access sites.
ABBREVIATIONS:
- dTRA
- distal transradial access
- FT
- fluoroscopy time
- pTRA
- proximal transradial access
- RAO
- radial artery occlusion
- TFA
- transfemoral access
- TRA
- transradial access
- US
- ultrasound
Neuroendovascular procedures have traditionally been performed using transfemoral access (TFA). Transradial access (TRA) recently gained popularity due to its lower rate of access site complications, quicker recovery time, and greater patient satisfaction.1 However, TRA is not without complications, including radial artery occlusion (RAO), hematoma, vasospasm, pseudoaneurysm, and arteriovenous fistula.2 Distal transradial access (dTRA) with puncture of the radial artery in the anatomic snuffbox may be safer than proximal transradial access (pTRA) in the forearm.3 The former is distal to the origin of the superficial palmar arch, lowering the risk of hand ischemia with RAO, and preserves the proximal radial artery for future interventions. It also affords shorter time to achieve hemostasis and improved ergonomics for both the patient and the operator.4 The latter may be especially beneficial in left-sided approaches and in patients who have limited supination.
Although numerous reports on the safety and efficacy of dTRA for coronary angiography and percutaneous coronary interventions are available, data regarding this approach for neuroendovascular procedures are sparse and have not been reviewed. The goal of this study was to perform a systematic review and meta-analysis of dTRA for cerebral angiography and neurointerventions to determine the success and complication rates of this approach.
MATERIALS AND METHODS
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.5
Search Strategy
We performed a comprehensive search of the literature as of August 21, 2020, using PubMed, Scopus, and EMBASE with the following keywords: (“distal radial” OR “distal transradial” OR “snuffbox”) AND (“cerebral” OR “neuroendovascular” OR “neurointervention”).
Selection Criteria
Studies were included if the authors reported original data regarding their outcomes performing distal transradial access for diagnostic cerebral angiography or neurointerventions. Only series of at least 10 patients were considered.
Data Extraction
A standardized form was used to extract the following data from the included studies: 1) number of patients undergoing the dTRA approach, 2) mean age, 3) proportion of diagnostic and interventional procedures, 4) success rate and reasons for failures, 5) complication rate and nature of complications, 6) use of ultrasound (US), 7) use of the Barbeau or Allen test, 8) method for achieving hemostasis, 9) mean radial artery diameter in the snuffbox, 10) sheath used, 11) mean number of vessels catheterized (diagnostic procedures), 12) mean fluoroscopy time (FT) (diagnostic procedures), and 13) contrast dose (diagnostic procedures). Data were extracted in duplicate by 2 authors (M.S.J. and H.H.), and all inconsistencies were resolved with discussion. In cases of missing data, the corresponding authors were contacted for clarification. The authors of 1 study provided details regarding additional cases that met the inclusion criteria, which were included in the quantitative analysis.6
Critical Appraisal
The methodologic quality of the studies was assessed using a previously described version of the Newcastle-Ottawa Scale modified for case series.7 Studies were evaluated in 4 domains: selection, ascertainment, causality, and reporting. Risk of publication bias across studies was evaluated using a funnel plot.
Statistical Analysis
Pooled estimates for outcomes were calculated using random-effects models and are represented by forest plots. The primary outcome was the proportion of procedural success, which was defined as catheterization of the intended vessels and completion of the angiogram or intervention without conversion to pTRA or common femoral access. Secondary outcomes included complication rates, defined as major or minor. Major complications included symptomatic RAO, pseudoaneurysm or arteriovenous fistula formation, and hematoma requiring transfusion. Minor complications included minor bleeding, asymptomatic RAO, and local pain or numbness extending beyond the duration of the procedure. Heterogeneity was evaluated with the I2 statistic. Outliers were identified using the Grubb test with a 5% significance level. Meta-regression models were developed to determine the associations between routine use of US and procedural success as well as complications.
RESULTS
Search Results
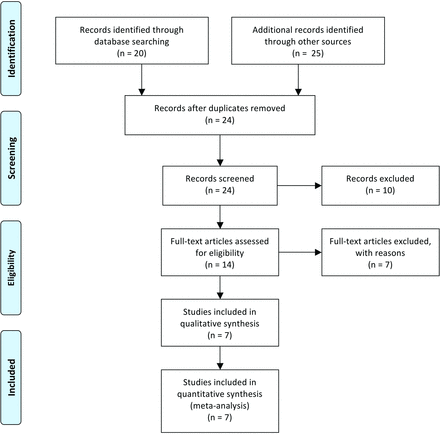
A total of 7 studies6,8⇓⇓⇓⇓-13 met the inclusion criteria and were included in the meta-analysis (Fig 1). All 7 studies were retrospective case series, and none of them compared outcomes associated with dTRA and pTRA or TFA. The methodologic quality of the 7 studies is described in Table 1.
PRISMA flow diagram.
Assessment of methodologic quality of the 11 included studies using criteria described by Murad et al7
Description of Studies and Procedural Characteristics
The 7 included studies comprised 348 (75.8%) diagnostic cerebral angiograms and 111 (24.2%) interventions. Three studies included only diagnostic angiograms,9,12,13 one included only interventions,10 and 3 combined both.6,8,11 Details of the interventions are provided in Table 2. The mean ages in each study ranged between 52 and 64.4 years. There was a slight female predominance (58.8%). The modified Allen test was routinely used in 1 study,9 but this did not preclude the authors' use of dTRA. Five studies reported routinely using US to guide arterial puncture,6,8,10,12,13 while 1 study used it in approximately 20% of cases9 and another did not use US at all.11 The mean diameter of the radial artery in the anatomic snuffbox was reported in 3 studies9,10,13 and ranged from 2.19 to 2.4 mm.
Details of each study
Procedural Success
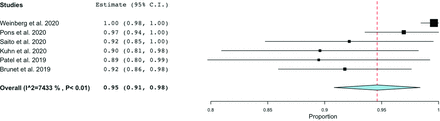
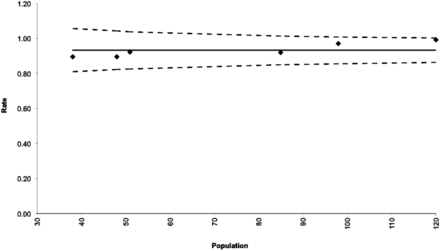
Success rates ranged from 20% to 100%. The 20% success rate reported by Goland et al11 was determined to be a statistically significant (P < .05) outlier and was removed from the analysis. As shown in Fig 2, the pooled success rate was 95% (95% CI, 91%–98%), though there was significant heterogeneity (I2 = 74.33, P < .01). Routine US use was associated with procedural success (OR = 1.41; 95% CI, 0.98–2.02), though this approached but did not reach statistical significance (P = .061). Reasons for conversion to pTRA or TFA included an inability to cannulate the radial artery, vasospasm, the presence of arteria lusoria, the presence of a radial artery loop, and lack of catheter support in the aortic arch. All 6 success rates were within the 95% confidence interval of the funnel plot for publication bias (Fig 3).
Forest plot demonstrating the pooled procedural success rate.
Funnel plot depicting the success rates for the 6 studies included in the pooled rate. The solid line represents the pooled success rate, and the hashed lines indicate its 95% confidence interval.
Successful selective catheterization of specific vessels was described in 2 studies.6,9 Pons et al6 reported decreasing success rates in selecting the right ICA (97%), left ICA (93.5%), and left vertebral artery (82%). Saito et al9 also reported decreased success in catheterizing the left ICA.
Complications
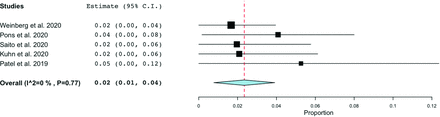
Five studies reported access-related complication rates.6,8⇓-10,12 No major complications were experienced in any of the series. The incidence of minor complications ranged from 1.7% to 5.9%. As shown in Fig 4, the pooled complication rate was 2% (95% CI, 1%–4%), and there was low heterogeneity (I2 = 0, P = .77). Routine US use was not associated with access-related complications (OR = 1.00; 95% CI, 0.96–1.05; P = .829). Of the 10 complications reported, hematoma was the most common (40%), followed by pain or numbness (30%), radial artery spasm (10%), dissection (10%), and asymptomatic RAO (10%). Only 1 study6 reported the rate of procedural complications (6.5%), which were related to aneurysm treatment in 2 patients and carotid stent placement in one.
Forest plot demonstrating the pooled rate of minor complications.
Procedural Details
Each study that included interventions described the use of 6F sheaths, while 5F sheaths were used for diagnostic cerebral angiograms with the exception of 1 study9 in which 4F sheaths were used. The use of Simmons type 1 or 2 catheters was described for diagnostic angiography in 4 studies.6,9,11,12 For interventions, long sheaths were described in 2 studies6,10 and included 6F 9-cm sheaths, including the Shuttle (Cook) and Ballast (Balt), as well as the AXS Infinity LS (Stryker Neurovascular). Kuhn et al10 also reported the use of Fubuki guide catheters (Asahi Intecc) in a sheathless fashion. Alternatively, 5F or 6F guide or intermediate catheters were placed directly through a short radial sheath.6,11 The mean/median number of vessels catheterized ranged from 1.5 to 5. FTs for diagnostic procedures could be combined from 4 studies (n = 285),8,9,12,13 yielding a mean FT of 13.53 [SD, 8.82] minutes. Contrast doses for diagnostic procedures were available in 3 studies,8,9,12 producing a combined mean of 74.9 [SD, 35.6] mL. All 7 studies described their methods for hemostasis. The PreludeSYNC DISTAL Radial Compression Device (Merit Medical) was the most common (4 studies), while the Safeguard Radial Compression Device (Merit Medical), the TR BAND Radial Compression Device (Terumo), and the Stepty P compression bandage (NICHIBAN) were used in 1 study each. One study did not use a hemostatic device.
DISCUSSION
Following the publication of multiple studies in the cardiology literature demonstrating the improved safety of TRA compared with TFA,14⇓⇓-17 the radial artery is becoming increasingly used for neurointerventional procedures. Multiple authors have reported high procedural success rates using TRA not only for diagnostic angiograms but also for interventions.18,19 TRA also affords direct access to the ipsilateral vertebral artery and easier vessel catheterization in type III aortic arches. dTRA has the same benefits as pTRA, with the potential advantages of reduced risk of hand ischemia, improved ergonomics, preservation of the proximal radial artery for future endovascular procedures or bypass, and shorter time to achieve hemostasis. While the safety and efficacy of dTRA have been established for coronary angiography and intervention,20 less evidence supports its use in cerebral angiography and neurointerventions. The latter requires more distal access in increasingly tortuous vessels, meriting its own study. Although success and complication rates associated with pTRA for neurointervention have been reviewed,2 dTRA requires a separate investigation due to its unique features. These include the smaller diameter, angled course in the snuffbox, propensity for vasospasm, and greater distance from the supra-aortic vessels of the distal radial artery. We found a high overall rate of procedural success and a low complication rate with dTRA, suggesting that it may be a useful addition to the neurointerventionalist's armamentarium. The lack of any direct comparison with pTRA in the literature precludes any conclusion regarding the superiority of one approach over the other.
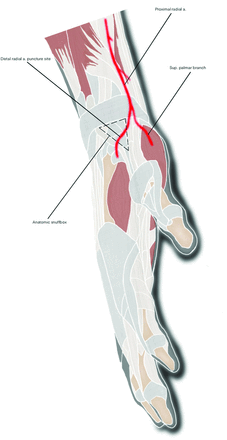
The puncture site for dTRA is in the proximal anatomic snuffbox (Fig 5), which is a triangular depression bounded by the tendons of the abductor pollicis longus and extensor pollicis brevis muscles laterally and the tendon of the extensor pollicis longus medially. The scaphoid and trapezium bones form the floor of the snuffbox. Here, the radial artery courses in a medial-to-lateral direction and continues as the deep palmar arch.
Diagram of the distal radial artery puncture site in the wrist and its proximal course superimposed on the surrounding anatomic structures. a indicates artery; sup, superficial.
Summary of Evidence
We identified a high pooled success rate of 95% in this study, which is comparable with the rates described in the cardiology literature. It is also similar to the 4.8% rate of crossover to TFA in a meta-analysis of pTRA for coronary interventions.15 In a meta-analysis of 5 studies with 3209 patients undergoing dTRA, Hamandi et al20 identified a nearly identical success rate of 94.7%. A separate meta-analysis of 4212 patients yielded similar results, demonstrating a 95.4% success rate.21 The inability to use dTRA can arise from various causes and steps in the procedure. Failure to cannulate the distal radial artery may occur due to hypoplasia or vasospasm from multiple punctures. A minimum artery diameter of 2 mm has been described, but little empiric evidence is available to support this. All 3 studies reporting the distal radial artery diameter obtained mean measurements of >2 mm, and Brunet et al13 did not identify a difference in radial artery diameter between the proximal and distal segments. US guidance may reduce the number of attempts required for arterial cannulation. Although the distal radial artery is usually palpable in the snuffbox, US can help ensure a single-wall puncture and visualize the course of the artery. The radial artery travels from medial to lateral in the snuffbox, requiring a 30°–45° angulation of the needle.22 The Radial Artery Access with Sonography Trial (RAUST) demonstrated a reduced number of attempts and shorter time to access with US guidance, though this was performed for pTRA.23 Six of the 7 studies in this review described the use of US, and meta-regression demonstrated an association between US and procedural success that neared statistical significance.
Vasospasm may also preclude dTRA, which was a frequently cited reason for access failure in this review. Slow injection of 2.5 mg of verapamil and 200 µg of nitroglycerin is commonly performed once access is obtained to avoid vasospasm around the sheath. Longer sheaths can also bypass the proximal radial artery where vasospasm is usually encountered. However, vasospasm may also be encountered on initial cannulation of the artery, especially with multiple punctures. In our experience, this can sometimes be avoided by infiltrating the periarterial tissues with a mixture of 1 mL of lidocaine and 200 µg of nitroglycerin before puncture. Following successful sheath placement, the remainder of the procedure is performed in a manner similar to pTRA, with potential reasons for conversion to TFA including the presence of a radial artery loop, arteria lusoria configuration, and lack of catheter support in the aortic arch. The latter may be particularly relevant for dTRA because of the approximately 5 cm of extra distance from the puncture site to the target vessel.
Complications were rare, minor, and self-limiting, findings similar to those in prior reports. Hamandi et al20 identified low rates of various complications with dTRA, ranging between 0.11% and 2.3%. In addition, RAO was lower with dTRA compared with pTRA.20 Park et al24 found a 2.2% minor complication rate with noncoronary and noncerebral interventions, which is almost identical to our pooled complication rate of 2%. These rates are lower than those of TFA, which is associated with a 2.8%–5.1% complication rate.25 Furthermore, many of these were major complications such as retroperitoneal hematoma, arteriovenous fistula, and pseudoaneurysm. Our 2% complication rate was similar to the 2.75% minor complication rate associated with pTRA.2 However, major complications have been reported with pTRA, including symptomatic RAO following carotid artery stent placement.26 Only 1 case of RAO was reported in this review, which was asymptomatic. This may underestimate the true incidence, however, given that follow-up US or angiography was not routinely performed. Minimizing the volume of air in the compression band needed to achieve hemostasis can reduce the risk of RAO.13 This follows the principles of the patent hemostasis technique, which is associated with lower rates of RAO.27
In our experience with the PreludeSYNC DISTAL, typical volumes range between 5 and 8 mL, which enable rapid hemostasis and discharge following elective diagnostic procedures. The time to achieve hemostasis may be shorter with dTRA than with pTRA.28 Overall, the lack of hand ischemia in any of the patients in this review can be explained by the location of the access site distal to the origin of the superficial palmar arch, which anastomoses with the ulnar artery and the deep palmar arch. Hematomas composed approximately half of the complications, which did not require transfusion. The radial artery in the snuffbox is superficial and easily compressible against the scaphoid bone. Prolonged pain was another minor complication, and both cases were self-limited. Patients may experience puncture site pain during the procedure, which is usually due to vasospasm. Typically, this resolves with conclusion of the procedure and removal of the sheath. US guidance could lower the rate of complications by reducing the number of arterial punctures, but we did not find an association between routine US use and the complication rate, likely due to the low pooled complication rate and relatively small sample size. Overall, the results of this review suggest that dTRA is very safe for both diagnostic procedures and interventions.
Fluoroscopy times for diagnostic angiograms performed with dTRA were slightly longer compared with prior reports using pTRA (6.5–10.3 minutes).29,30 However, direct comparison is difficult without adjusting for the number of vessels catheterized and operator experience. As mentioned previously, the additional length to the aortic arch with dTRA theoretically could make the procedure more difficult and increase FTs. Studies directly comparing FT between pTRA and dTRA are warranted.
Limitations
Limitations include the small number of studies, which reflects the relatively late adoption of radial access in the neurointerventional field. All these studies were retrospective, and the decision to perform dTRA could have introduced selection bias. In addition, there were relatively few interventions included (n = 111). Success rates are highly dependent on operator experience, and a substantial learning curve for dTRA exists. Therefore, various levels of experience could have introduced variability into the success rate, which we attempted to account for with a random-effects model. This may have contributed to the significant heterogeneity of this outcome. Several studies did not report each variable that we collected, which we tried to address by contacting the original authors. Procedural complications (ie, occurring after obtaining access) were sparsely reported but are important for assessing the overall safety of the approach. Only 2 studies reported their success in selecting specific vessels, which is an important measure of the efficacy of dTRA, given that left ICA and vertebral artery catheterization is more difficult with this approach. An additional limitation is the lack of any comparison with pTRA or TFA.
Future Directions
Additional series regarding the safety and efficacy of dTRA for cerebral angiography and neurointervention are needed because the current literature comprises a small number of centers with operators who may have had prior experience performing radial access. In 1 study, all operators had performed at least 50 angiograms with pTRA.13 Therefore, caution should be used when extrapolating the results of this meta-analysis. To this end, characterization of operators' learning curves transitioning to dTRA may be informative. Studies directly comparing pTRA and dTRA may offer insight regarding any superiority of one approach over the other. The ongoing DIStal Versus COnventional RADIAL Access for Coronary Angiography and Intervention (DISCO) radial trial (clinicaltrials.gov Identifier: NCT04171570) will determine the success and complication rates associated with each radial artery access site for coronary procedures. Similar trials for neurointervention are warranted. As more operators adopt dTRA, the technique will likely be refined and even greater success rates will be realized. The development of catheters specifically designed for transradial neurointervention may also improve the efficacy of the approach.
CONCLUSIONS
dTRA is a safe and effective option for diagnostic cerebral angiography and neurointervention that has distinct advantages compared with pTRA and TFA. However, the literature is mostly limited to small, single-institution case series and diagnostic procedures. Additional studies are required with large sample sizes, greater proportions of interventions, prospective enrollment, and direct comparisons with other approaches.
Footnotes
Disclosures: Priyank Khandelwal—RELATED: Consulting Fee or Honorarium: American Academy of Cardiology, Comments: paid $2000 honoraria as a speaker; UNRELATED: Grants/Grants Pending: pending, feasibility of the Infinity Catheter for transradial neurology procedures, Comments: submitted, under review. Roger B. Pons—UNRELATED: Payment for Development of Educational Presentations: Merit Medical, Comments: presentations for educational purpose.
References
- Received September 14, 2020.
- Accepted after revision November 11, 2020.
- © 2021 by American Journal of Neuroradiology