Abstract
SUMMARY: Hydrocephalus is one of the earliest manifestations of mucopolysaccharidosis I-Hurler syndrome, and delayed treatment of hydrocephalus can lead to neurocognitive delay or even death. Optic nerve sheath diameter has been established as a noninvasive measurement to detect elevated intracranial pressure. This study aimed to establish correlations between optic nerve sheath diameter and opening pressure. Forty-nine MR images and opening pressures in patients with mucopolysaccharidosis I-Hurler syndrome were retrospectively reviewed from 2008 to 2020. The optic nerve sheath diameter was measured 3 mm posterior to the posterior margin of the globe (retrobulbar) and 10 mm anterior to the optic foramen (midpoint segment), and the average was taken between the 2 eyes. Opening pressure was measured with the patient in the lateral decubitus position with controlled end-tidal CO2 on the same day as the MR imaging. The average retrobulbar optic nerve sheath diameter was 5.33 mm, higher than the previously reported measurement in healthy controls, in patients with idiopathic intracranial hypertension, and there was a positive correlation between age and the optic nerve sheath diameter measured at the retrobulbar or midpoint segment (retrobulbar segment, R2 = 0.27, P < .01; midpoint segment, R2 = 0.20, P < .01). However, there was no correlation between retrobulbar or midpoint segment optic nerve sheath diameter and opening pressure (retrobulbar segment, R2 = 0.02, P = .17; midpoint segment, R2 = 0.03, P < .12). This study shows a higher average optic nerve sheath diameter in patients with mucopolysaccharidosis I-Hurler syndrome than in healthy controls regardless of the location of the measurement. However, the degree of optic nerve sheath dilation does not correlate with opening pressure, suggesting that increased optic nerve sheath diameter is an ocular manifestation of mucopolysaccharidosis I-Hurler syndrome itself rather than a marker of elevated intracranial pressure.
ABBREVIATIONS:
- GAG
- glycosaminoglycan
- ICP
- intracranial pressure
- IIH
- idiopathic intracranial hypertension
- MPSIH
- mucopolysaccharidosis type I-Hurler syndrome
- ONSD
- optic nerve sheath diameter
- OP
- opening pressure
Mucopolysaccharidosis type I-Hurler syndrome (MPSIH) is a progressive disorder caused by a deficiency of alpha-L-iduronidase, critical in glycosaminoglycan (GAG) metabolism. Hydrocephalus is one of the earliest manifestations of MPSIH, and its pathophysiology is theorized to be due to deposits of GAG and/or skull abnormalities.1 One study reported that 30.6% of patients developed hydrocephalus before hematopoietic stem cell transplantation and 16.5% of patients required shunt placement.2 However, patients with MPSIH may also have profound brain atrophy resulting in ventriculomegaly, thus making the diagnosis of hydrocephalus challenging.1
Optic nerve sheath diameter (ONSD) has been established as a reliable predictor for patients suspected of having idiopathic intracranial hypertension (IIH). The subarachnoid space surrounding the optic nerve expands in response to elevated intracranial pressure (ICP), due to its connection to the CSF. It has been established that patients with IIH have higher ONSDs than healthy controls.3⇓-5 One case series demonstrated elevated ONSD in patients with MPSIH compared with a healthy pediatric cohort, but it was unclear whether this finding was due to elevated ICP or ocular manifestations of MPSIH.6
In this study, the average ONSD and the opening pressure (OP) were examined and analyzed to see whether the ONSD could predict elevated ICP in patients with MPSIH.
MATERIALS AND METHODS
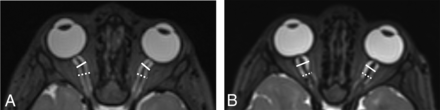
All patients with MPSIH who were treated with stem cell transplantation between the 2008 and 2020 at the University of Minnesota were retrospectively reviewed. Patients who had concomitant brain MR imaging studies under sedation and lumbar punctures with OP measurements during the pretransplant evaluation were identified. Patients with ventriculoperitoneal shunts and those without documented OPs were excluded. The ONSD was measured perpendicular to the optic nerve on axial T2-weighted images (either 1-mm reconstructed images when 3D T2-weighted images were obtained or 3 mm when 2D T2-weighted turbo spin-echo images were obtained) in 2 locations, approximately 3 mm posterior to the optic disc (retrobulbar segment) and approximately 10 mm anterior to the orbital apex (midpoint segment) (Fig 1). Measurements from both eyes were obtained, and the average of each patient was considered in the analysis. Although the institutional imaging protocol has changed and occasionally images were obtained outside our institution, the measurements could be easily and reliably obtained in all cases. The consensus review was achieved between a neurosurgery resident and a neuroradiologist attending. OP was obtained with the patient under sedation via lumbar puncture in the lateral decubitus position with 25–40 mm Hg of end-tidal CO2. The results were reported as mean and 95% confidence interval. The ONSD was plotted versus age, and the OP was plotted versus ONSD. Correlation coefficients were reported.
A, Axial T2-weighted MR image of the brain shows white sold lines indicating the retrobulbar ONSD and white dashed lines indicating the midpoint of the ONSD. This patient’s OP was elevated at 31 cm H2O, while the average ONSD was 5.8 mm at the retrobulbar area and 5.4 mm at the midpoint. B, Axial T2-weighted MR image of the brain shows white solid lines indicating the retrobulbar ONSD and white dashed lines indicating the midpoint ONSD. The courses of the optic nerves in this patient are more tortuous than those in the patient shown in A. This patient’s OP was normal at 17 cm H2O, while the average ONSD was 8.3 mm at the retrobulbar area and 6.5 mm at the midpoint.
RESULTS
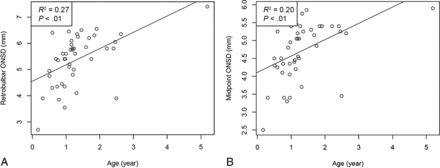
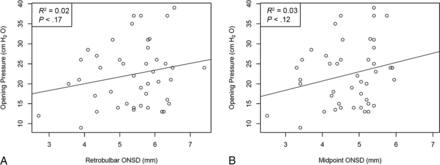
Sixty-six patients were reviewed retrospectively, and 6 patients with shunts were excluded from the analysis. Sixteen patients who never had documented OP were excluded. A total of 44 measurements of the OP were recorded with an average of 22.3 cm H2O (95% CI, 0.1–24.5 cm H2O). Forty-four MR imaging brain scans were examined for the ONSD: The retrobulbar segment average was 5.33 mm (95% CI, 5.07–5.58 mm), and the midpoint segment average was 4.68 mm (95% CI, 4.46–4.90 mm; P < .01). The average ONSD was further stratified into 3 age groups, 0–1 year (n = 16), 1–2 years (n = 22), and >2 years (n = 6) as shown in the Table. There was an increase in the average ONSD measured both at the retrobulbar and midpoint segments from 0–1 to 1–2 years: 4.67–5.75 mm (P < .01) and 4.09–5.06 mm (P < .01) (Table). There was a statistically significant correlation between age and the ONSD at the retrobulbar segment (R2 = 0.27, P < .01) and midpoint (R2 = 0.20, P < .01) (Fig 2). However, while there was a trend toward increased ICP with increased ONSD at the retrobulbar segment (R2 = 0.02, P = .17) and midpoint (R2 = 0.03, P = .12), this did not reach statistical significance (Fig 3).
Number of ONSDs, average ONSD, and 95% CI stratified by age
A, Scatterplot between retrobulbar ONSD and age shows a positive correlation (R2 = 0.27, P < .01). B, Scatterplot between the midpoint ONSD and age shows a positive correlation (R2 = 0.20, P < .01).
A, Scatterplot between the retrobulbar ONSD and OP does not show a correlation (R2 = 0.02, P = .17). B, Scatterplot between the retrobulbar ONSD and OP does not show a correlation (R2 = 0.03, P = .12).
DISCUSSION
MPSIH is a severe lysosomal storage disease, and one of the earliest manifestations is hydrocephalus. Delayed recognition and treatment can lead to neurocognitive developmental delay or even mortality.7,8 Moreover, it has been previously shown that the average OP is elevated in the MPSIH cohort compared with the healthy pediatric population.9 ONSD has been recognized as a useful, noninvasive measurement to detect elevated intracranial pressure. The subarachnoid space between the optic nerve sheath and the optic nerve widens as the intracranial pressure increases due to its connection to the intracranial CSF. Multiple studies have examined its utility in patients with suspected IIH and have established the average values in pediatric populations and the pediatric IIH populations.3⇓-5,10
However, there is controversy regarding the best location along the optic nerve for the ONSD assessment, and the technique and measurements often vary from study to study. While most studies report retrobulbar measurements, some authors strongly advocate midpoint measurements. One reason is that histologic studies have shown that the dura close to the globe is more capacious and the dura close to the optic foramen becomes tighter. Eye movement can alter the shape and diameter of the loose dura that is close to the globe. Another reason is that the retrobulbar dura can also be affected by the ocular pathology itself and lead to an increase in the ONSD.11 With greater retrobulbar ONSD in this study, it confirmed the previous finding that retrobulbar ONSDs are larger than midpoint ONSDs due to the larger subarachnoid space in the immediate retrobulbar region than in the midpoint segment posterior to the globe (Table).
Janthanimi and Dumrongpisutikul12 reported a retrobulbar ONSD of 4.81 mm for 0–1 year, 5.0 mm for 1–2 years, 4.9 mm for 2–3 years, and 5.2 mm for 3–4 years in healthy patients. Shofty et al13 measured the midpoint ONSD in healthy children and children with IIH, stratified by age groups. They reported that among children younger than 3 years of age, an average of 3.1 mm was measured in the healthy cohort compared with 4.35 mm in the IIH cohort. Unfortunately, there are no reported reference measurements in the literature in patients with MPSIH. There are prior case series reporting optic nerve edema in more than one-half of the cohort, with 1 study reporting an average retrobulbar ONSD of 5.25–6.71 mm, markedly more elevated than in a healthy population, and another study of 66 patients with the diagnosis of mucopolysaccharidosis I, II, and VI, comparable with the mean ONSD of 5.33 mm in our study.6,14,15
Contrary to the prevalence of increased ONSD, 1 study reported 30.6% of patients developing hydrocephalus before treatment, with only 16.5% eventually requiring shunt placement.2 Therefore, it was unclear whether the mechanism of the increase in ONSD is intracranial hypertension or GAG deposits in the subarachnoid space or a combination of both.6
In this study, we examined pediatric patients who did not have shunts and reported average retrobulbar ONSDs of 5.33 mm, which is higher than the previously reported mean in healthy cohorts and patients with IIH.13 When the measurements were further broken down by age group, the ONSD in our cohort was larger than that in the study of Janthanimi and Dumrongpisutikul12 in every patient age group except in the 0- to 1-year group (Table). These findings re-demonstrate the previous finding that the ONSD in patients with MPSIH is larger than that in healthy cohorts. The increase in the ONSD with age also illustrates a similar pattern observed in healthy cohorts, but it is unclear whether the relationship between ONSD and age is a manifestation of normal physiological change or further deposits of GAG (Fig 2).13 Most interesting, in our study, a sharp increase in the ONSD was observed from 0–1 year to 1–2 years; perhaps it can be partially explained by a rapid increase in the size of the optic nerve itself in the first 2 years of life.16
The normal range of OP in the pediatric population has been a subject of debate in the literature. Initially, the proposed normal upper limit of OP in healthy children was <20 cm H2O, and it was not until 1994 when Ellis17 performed serial lumbar punctures with the patient in the lateral decubitus position in patients with leukemia and found a mean OP of 19 cm H2O and a normal range of 10–28 cm H2O. Recent studies have reported a mean OP of 19.6 cm H2O, with a range of 11.5–28 cm H2O, which further suggested that the normal upper limit of OP should be 28 cm H2O.18,19 In fact, intracranial pressure of >28 cm H2O is one of the diagnostic criteria for IIT; the estimated incidence of pediatric IIH is 0.6–0.7 cases per 100,00 children.20,21 The average OP in our patient cohort was elevated at 22.3 cm H2O compared with the normal mean of <20 cm H2O in a healthy population.17⇓-19 Although there is no established normal OP in the MPSIH population, the OP in this study is comparable with the results in a previous study in which a mean of 24 cm H2O was reported.9 Contrary to the population without MPSIH, there was no statistically significant correlation found between the increased ONSD and increased OP measurements. As illustrated in Fig 1, patients with large ONSDs may, paradoxically, have low OP and vice versa. These findings challenged the notion that the increase in ONSD in patients with MPSIH is purely driven by elevated intracranial pressure (Fig 3). It is possible that GAG deposition and/or inflammatory changes play a role in this finding. Therefore, while elevated ICP is common in MPSIH, increased ONSD does not appear to be an accurate predictor of elevated intracranial pressure.
CONCLUSIONS
ONSD has been established as a noninvasive tool to suggest elevated intracranial pressure, but its adoption in patients with MPSIH is questionable. This study demonstrates higher ONSDs in patients with MPSIH than in healthy controls, but it does not accurately predict increased ICP.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- Received September 23, 2022.
- Accepted after revision December 5, 2022.
- © 2023 by American Journal of Neuroradiology